Metagenomics
Metagenomics is analysis of the genetic material of many organisms present in an environmental sample. It allows project managers to survey the local microbial community to make more informed remediation-related decisions. This approach is not targeted, but also not quantitative nor a complete assessment, especially for low-abundance microorganisms. However, it is a robust tool for assessing overall microbial community composition and observing its changes throughout the project lifecycle as conditions change and treatment technologies are implemented.
Contents
- 1 Introduction
- 2 Advantages
- 3 Limitations
- 4 Assessing Changes in Microbial Community Composition and Dynamics
- 5 Investigating Potential Interactions Between Microbial Groups
- 6 Identifying Novel and Related Functional Genes
- 7 Selecting Sample Locations
- 8 Sample Collection, Preservation, and Shipping
- 9 Summary
- 10 References
- 11 See Also
Related Article(s):
- Molecular Biological Tools - MBTs
- Quantitative Polymerase Chain Reaction (qPCR)
- Stable Isotope Probing (SIP)
- Compound Specific Isotope Analysis (CSIA)
CONTRIBUTOR(S): Dora Ogles-Taggart and Dr. Brett Baldwin
Key Resource(s):
Introduction
Metagenomics is defined as the analysis of th genome (i.e., the complete DNA sequence) of multiple organisms. In environmental remediation applications, metagenomics refers to the analysis of the collective genomes of all microorganisms present in a soil, groundwater, or sediment sample. Metagenomics is a powerful tool for surveying the composition of the microbial community, assessing biodiversity, and gaining insight into interactions between different microbial groups. Metagenomics is most often employed to answer the questions: What organisms are present? How many different types of organisms are present? How might they interact?
Advantages
- Cultivation independent: Less than 1% of bacteria can be cultivated (grown) in the laboratory<[1]. Like other molecular biological tools (MBTs), metagenomics eliminates the need to grow organisms in the laboratory, thus eliminating the biases associated with traditional, cultivation-based methods like plate counts.
- Non-Targeted: In theory, the entire genetic composition of the sample can be extracted, sequenced, and analyzed to give a profile representing all organisms and potential functions of the microbial community.
- Therefore, metagenomics can retrieve unknown gene sequences leading to the “discovery” of novel microorganisms and functional genes.
- No a priori knowledge of the microbial community composition or function is needed for the analysis.
Limitations
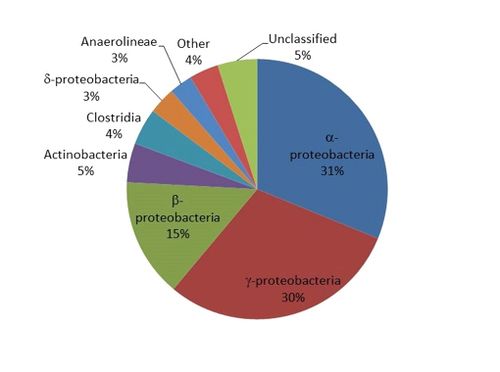
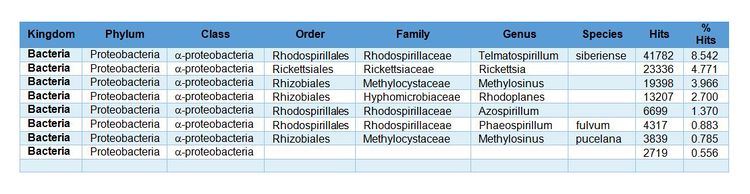
- Not quantitative: Results are expressed as the number of DNA sequences (reads) or percent of reads identified. For example, a sample analysis shows that ~31% of the DNA sequences belonged to the Class α-proteobacteria (Fig. 1).
- Not representative of low abundance microorganisms: Sequencing may miss important microorganisms present in the sample at low concentrations[2]. In other words, the absence of a microorganism from the dataset is not conclusive evidence that the microorganism is absent from the sample.
- Functional genes usually not analyzed: While applicable to functional genes, metagenomics is often limited to phylogeny -- analysis of the 16S rRNA gene which is used to identify organisms with the goal of determining what organisms are present in an environmental sample. A specific function, such as the ability to biodegrade a particular contaminant, often cannot be reliably inferred from identification of the microorganism. In other words, answering the question “What organisms are present?” does not always answer the question “What can they do?”
Assessing Changes in Microbial Community Composition and Dynamics
Metagenomics can be a tool for comprehensive examination of the subsurface microbial community responses to perturbations (changes in conditions) such as contaminant release or treatment technology. For example, metagenomics was used to test groundwater at the Oak Ridge Environmental Remediation Sciences Program Field Research Center (FRC) that is highly acidic and contaminated with uranium, nitrate, technetium, and a variety of organic compounds[3]. Results indicated that a simple microbial community existed despite the contamination, dominated by γ- and β-proteobacteria. Results also indicated that some members of the microbial community adapted to contain specific genes involved in transporting chromate out of the cell. They were resistant to severe heavy metal contamination using the ChrAB gene which encodes a protein that transports chromate out of the cell.
Metagenomics was also used to investigate the impact and potential biodegradation of ~4.1 million barrels of oil released in the Gulf of Mexico during the Deepwater Horizon incident in 2010. Results revealed that bacteria of the order Oceanospirillales and alkane degraders initially enriched in the dissolved plume[4] were supplanted by Cycloclasticus and Colwellia and later by methylotrophic bacteria[5][6]. The results suggested that biodegradation contributed to the loss of alkanes and methane from the Deepwater Horizon plume.
Investigating Potential Interactions Between Microbial Groups
Metagenomics has also been useful in elucidating potential syntrophic interactions in mixed cultures that generally allow for more robust biodegradation than observed by the corresponding pure cultures. Syntrophism is a partnership between two or more groups of organisms often where the metabolism of one group supports that growth of the other groups (e.g., cross-feeding). Syntrophic partnerships are common in anaerobic systems. For example, fermenters breakdown organic substrates such as volatile fatty acids producing hydrogen which methanogens and organohalide-respiring bacteria (e.g., Dehalococcoides mycartyi) use as an energy source. Metagenomic analysis of the trichloroethylene (TCE) dechlorinating consortium enriched from the Alameda Naval Air Station (ANAS) revealed working syntrophic relationships between hydrogen producers (fermenters) and consumers (organohalide-respiring bacteria and methanogens). The results also suggested that interactions supply critical co-factors and aid in reductive dechlorination[7].
Identifying Novel and Related Functional Genes
In research settings, metagenomic libraries are combined with screening approaches (phenotype screening, substrate-induced gene expression profiling and metabolite expression profiling) in order to discover new classes of genes with known or new functions. In phenotype-based screening, a metagenomics DNA library is constructed from the metagenomes isolated from a contaminated site. The library is then screened for clones exhibiting the desired phenotype. For example, the phenotype screening approach has been used to identify a novel styrene monooxygenase from a soil metagenomic library[8]. However, success rates for function-based screening methods can be extremely low.
Sequence-based methods are also available for targeted metagenomics. Often with this approach, degenerate PCR primers based on consensus DNA sequences of known catabolic genes are used for direct amplification of the gene encoding enzymes of similar function from the environmental genome. For example, the degenerate primer based screening has been used to study the diversity of reductive dehalogenase genes[9] and aromatic dioxygenase genes[10].
Selecting Sample Locations
Below are a few guidelines for selecting sampling locations to aid in drawing conclusions from metagenomic data.
- Background: Samples from non-impacted background area can be compared with results from impacted areas to examine the impact of contamination on microbial community composition.
- Baseline: These samples are collected and analyzed prior to treatment as a baseline for evaluating changes in the microbial community in response to the remediation.
- Plume: These samples are collected from distinct zones within the source area or contaminant plume to reflect variations in contaminant concentrations, geochemical conditions, and other site-specific criteria.
Sample Collection, Preservation, and Shipping
Sampling procedures for metagenomics and transcriptomics analyses are straightforward and readily integrated into existing monitoring programs. Almost any type of sample matrix (soil, sediment, groundwater, on-site filters) can be analyzed. All samples should be shipped to the laboratory on ice (4°C) using an overnight carrier to minimize the potential for changes in the microbial community between collection and analysis.
Groundwater samples (typically 1 L) can be shipped directly to the laboratory or filtered in the field. For on-site filtration, groundwater is pumped through a Sterivex® or Bio-Flo® filter using standard low flow sampling techniques[11]. The groundwater may then be discarded appropriately. As with other sample types, filters should be shipped on ice (4°C) using an overnight carrier.
Summary
In environmental remediation applications, metagenomics is a powerful tool for surveying microbial community composition, assessing microbial biodiversity, and gaining insight into interactions between different microbial groups. Metagenomics is a non-targeted analysis meaning that no α priori knowledge is needed to generate a profile of the microbial community composition. Therefore, metagenomics is ideally suited for answering the question “How is there?” as a means of assessing the overall impacts of changes in conditions such as contaminant release or treatment technology on microbial community composition. However, metagenomics results are not quantitative and may miss important microorganisms present in low abundances.
References
- ^ 1.0 1.1 Amann, R.I., Ludwig, W., Schleifer, K.H., 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiology and Molecular Biology Reviews, 59(1), 143-169. Journal Article
- ^ Hazen, T.C., Rocha, A.M., Techtmann, S.M., 2013. Advances in monitoring environmental microbes. Current Opinion in Biotechnology, 24(3), 526-533. doi:10.1016/j.copbio.2012.10.020
- ^ Hemme, C.L., Deng, Y., Gentry, T.J., Fields, M.W., Wu, L., Barua, S., Barry, K., Tringe, S.G., Watson, D.B., He, Z., Hazen, T.C., 2010. Metagenomic insights into evolution of a heavy metal-contaminated groundwater microbial community. The ISME Journal, 4(5), 660-672.doi:10.1038/ismej.2009.154
- ^ Mason, O.U., Hazen, T.C., Borglin, S., Chain, P.S., Dubinsky, E.A., Fortney, J.L., Han, J., Holman, H.Y.N., Hultman, J., Lamendella, R., Mackelprang, R., 2012. Metagenome, metatranscriptome and single-cell sequencing reveal microbial response to Deepwater Horizon oil spill. The ISME Journal, 6(9), 1715-1727. doi:10.1038/ismej.2012.59
- ^ Kessler, J.D., Valentine, D.L., Redmond, M.C., Du, M., Chan, E.W., Mendes, S.D., Quiroz, E.W., Villanueva, C.J., Shusta, S.S., Werra, L.M., Yvon-Lewis, S.A., 2011. A persistent oxygen anomaly reveals the fate of spilled methane in the deep Gulf of Mexico. Science, 331(6015), 312-315. doi: 10.1126/science.1199697
- ^ Mason, O.U., Scott, N.M., Gonzalez, A., Robbins-Pianka, A., Bælum, J., Kimbrel, J., Bouskill, N.J., Prestat, E., Borglin, S., Joyner, D.C., Fortney, J.L., 2014. Metagenomics reveals sediment microbial community response to Deepwater Horizon oil spill. Isme Journal, 8(7), 1464-1475. doi:10.1038/ismej.2013.254
- ^ Brisson, V.L., West, K.A., Lee, P.K., Tringe, S.G., Brodie, E.L., Alvarez-Cohen, L., 2012. Metagenomic analysis of a stable trichloroethene-degrading microbial community. The ISME Journal, 6(9), 1702-1714. doi:10.1038/ismej.2012.15
- ^ Van Hellemond, E.W., Janssen, D.B. and Fraaije, M.W., 2007. Discovery of a novel styrene monooxygenase originating from the metagenome. Applied and Environmental Microbiology, 73(18), 5832-5839. doi: 10.1128/AEM.02708-06
- ^ Hug, L.A., Edwards, E.A., 2013. Diversity of reductive dehalogenase genes from environmental samples and enrichment cultures identified with degenerate primer PCR screens. Frontiers in Microbiology, 4, 341. doi: 10.3389/fmicb.2013.00341
- ^ Iwai, S., Chai, B., Sul, W.J., Cole, J.R., Hashsham, S.A., Tiedje, J.M., 2009. Gene-targeted-metagenomics reveals extensive diversity of aromatic dioxygenase genes in the environment. The ISME Journal, 4(2), 279-285. doi:10.1038/ismej.2009.104
- ^ Lebrón, C. A., Dennis, P., Acheson, C., Barros, N., Major, D., Petrovskis, E., Loffler, F.E., Ritalahti, K.M., Yeager, C.M., Edwards, E.A., Hatt, J.K., Ogles, D.M., 2014. Standardized procedures for use of nucleic acid-based tools - Recommendations for groundwater sampling and analysis using qPCR. Project ER-1561. Strategic Environmental Research Development Program, Arlington, VA. ER-1561