Difference between revisions of "Munitions Constituents"
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
Munitions Constituents (EM) are chemicals used in formulations as propellants, pyrotechnics, and explosives in weapon systems, munitions, and blasting agents. This article introduces these materials, major physical and chemical properties, and fate in the environment. Important chemical groups include nitroaromatics (e.g. 2,4,6-trinitrotoluene (TNT) and 2,4-dinitrotoluene (2,4-DNT), nitramines (e.g. hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetreazocine (HMX)), nitrate esters (e.g. nitroglycerin (NG), pentaerythritol tetranitrate (PETN)), and nitrocellulose (NC). Ammonium perchlorate (AP) is commonly used as a propellant in solid rock fuel and is addressed in a separate article on [[Perchlorate | perchlorate]]. Insensitive munitions (IM) are energetics in newer military explosives and are generally considered more stable than traditional explosives. | Munitions Constituents (EM) are chemicals used in formulations as propellants, pyrotechnics, and explosives in weapon systems, munitions, and blasting agents. This article introduces these materials, major physical and chemical properties, and fate in the environment. Important chemical groups include nitroaromatics (e.g. 2,4,6-trinitrotoluene (TNT) and 2,4-dinitrotoluene (2,4-DNT), nitramines (e.g. hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetreazocine (HMX)), nitrate esters (e.g. nitroglycerin (NG), pentaerythritol tetranitrate (PETN)), and nitrocellulose (NC). Ammonium perchlorate (AP) is commonly used as a propellant in solid rock fuel and is addressed in a separate article on [[Perchlorate | perchlorate]]. Insensitive munitions (IM) are energetics in newer military explosives and are generally considered more stable than traditional explosives. | ||
| − | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> | + | <div style="float:right;margin:0 0 2em 2em;">__TOC__</div> |
| − | |||
'''Related Article(s):''' | '''Related Article(s):''' | ||
| Line 18: | Line 17: | ||
| − | ''' | + | '''Contributor(s):''' [[Dr. Kevin Finneran]] and [[Dr. Robert Borden, P.E.]] |
Revision as of 20:11, 27 April 2022
Munitions Constituents (EM) are chemicals used in formulations as propellants, pyrotechnics, and explosives in weapon systems, munitions, and blasting agents. This article introduces these materials, major physical and chemical properties, and fate in the environment. Important chemical groups include nitroaromatics (e.g. 2,4,6-trinitrotoluene (TNT) and 2,4-dinitrotoluene (2,4-DNT), nitramines (e.g. hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetreazocine (HMX)), nitrate esters (e.g. nitroglycerin (NG), pentaerythritol tetranitrate (PETN)), and nitrocellulose (NC). Ammonium perchlorate (AP) is commonly used as a propellant in solid rock fuel and is addressed in a separate article on perchlorate. Insensitive munitions (IM) are energetics in newer military explosives and are generally considered more stable than traditional explosives.
Related Article(s):
- Metal(loid)s - Small Arms Ranges
- Munitions Constituents - Abiotic Reduction
- Munitions Constituents - Alkaline Degradation
- Munitions Constituents - Composting
- Munitions Constituents - Deposition
- Munitions Constituents - Dissolution
- Munitions Constituents - IM Toxicology
- Munitions Constituents – Photolysis
- Munitions Constituents - Soil Sampling
- Munitions Constituents - Sorption
- Munitions Constituents - TREECS™ Fate and Risk Modeling
- Passive Sampling of Munitions Constituents
Contributor(s): Dr. Kevin Finneran and Dr. Robert Borden, P.E.
Key Resource(s):
- EPA Federal Facilities Forum Issue Paper: Site Characterization for Munitions Constituents, EPA/505/S-11/001, 2012.[1]
Explosives and Propellants
Explosive materials are commonly classified according to the speed of the chemical reaction wave that propagates through the material. If the wave velocity is greater than the speed of sound (supersonic), the material is said to undergo detonation and is considered an explosive. If the wave propagation velocity is less than the speed of sound, the material is considered to undergo deflagration (rapid burning) and is often used as a propellant[1].
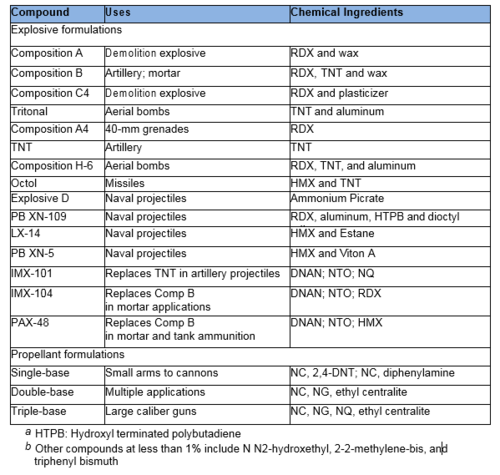
Common military explosives are mixtures consisting of one or more explosive compounds including trinitrotoluene (TNT), 1,3,5-hexahydro-1,3,5-trinitrotriazine or Research Development Explosive (RDX), octrahydro-1,3,5,7- tetranitro-1,3,5,7-tetrazocine or High Melting Explosive (HMX), and 2,4,6- trinitro-phenylmethylnitramine (tetryl) and ammonium picrate. However, the US military is replacing many of these materials with insensitive munitions (IM) to reduce risks of accidental detonation. IM materials will burn, rather than explode, when subjected to fast or slow heating, bullets, shrapnel, shaped charges, or the detonation of another nearby munition. Important components of IM include 2,4-dinitroanisole (DNAN), nitroguanidine (NQ), 3-nitro-1,2,4-triazol-5-one (NTO) and other traditional munitions components (RDX, HMX). We highlight common military explosives, propellants, and IM formulations in Table 1.
Propellant formulations often contain several components. The primary component is often nitrocellulose (NC), which is combined with other EM compounds including nitroglycerin (NG), NQ, DNT, HMX, burn rate modifiers, binders or plasticizers, and stabilizers. Gun propellants usually are single component based (e.g., NC), double based (e.g., NC and NG), or triple based (e.g., NC, NG, and NQ).
Physical and Chemical Properties
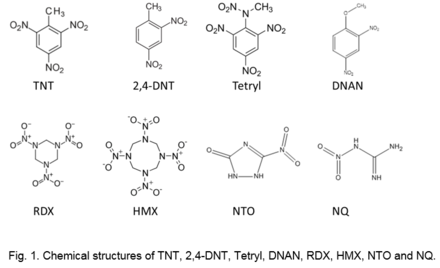
The structure (e.g., Fig. 1), as well as physical and chemical properties of explosive materials control their fate and transport in the environment (Table 2).
With the exception of NG, the major EM are solids at ambient temperatures (Table 2). Table 2 provides the molecular mass, aqueous solubility, Log octanol-water partition coefficient (Log Kow), and vapor pressure of common explosive materials. Although NG is a liquid, it is commonly used as a component of double- and triple-base propellants, with the solid polymeric NC. Aqueous solubility of EM varies dramatically between the different materials and can have an important influence on their mobility in the environment. Organic compounds with a high Kow are more likely to sorb to organic carbon in soil or bioaccumulate; however, EM tend to be high in nitrogen, and by definition, are strong oxidizing agents. EM materials tend to form crystals. The vapor pressure of these materials is relatively low, so volatilization is not an important removal mechanism for most EM.
| Explosive | CAS | Formula | Molecular Weight [g/mol] | Aqueous Solubility at 25°C [mg/L] | Log Kow | Vapor Pressure at 20°C
[mm Hg] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TNT | 118-96-7 | C7H5N3O6 | 227.13c | 130c | 1.60c | 1.99 E-4c | |||||
| RDX | 121-82-4 | C3H6N6O6 | 222.26d | 56.4i | 0.87d | 1 to 4 E-9d | |||||
| HMX | 2691-41-0 | C4H8N8O8 | 296.16a | 4.5i | 0.165i | 3.3E-14a | |||||
| Tetryl | 479-45-8 | C7H5N5O8 | 287.14a | 80a | 5.7E-9 (25°C)a | ||||||
| 2,4-DNT | 121-14-2 | C7H6N2O4 | 182.15a | 300 (22°C) f | 1.98f | 1.47E-4f | |||||
| 2,6-DNT | 606-20-2 | C7H6N2O4 | 182.15a | 180 (22°C)f | 1.72 or 2.10f | 5.67E-4f | |||||
| 2-ADNT | 35572-78-2 | C7H7N3O4 | 197.17a | 2800a | 4.0E-5a | ||||||
| 4-ADNT | 19406-51-0 | C7H7N3O4 | 197.17a | 2800a | 2.0E-5a | ||||||
| NTO | 932-64-9 | C2H2N4O3 | 130.08 | 2000m | 0.858m | ||||||
| DNAN | 119-27-7 | C7H6N2O5 | 198.13j | 213o, p | 1.58o, p | 1.E-4 (25°C)j | |||||
| NQ | 556-88-7 | CH4N4O2 | 104.07 | 4,400n | -0.89j
0.21l |
1.00E-9 (est)j | |||||
|
a = Thiboutout et al. (2002)[2], b = Pennington et al. (2006) Final Report[5], c = EPA Technical Fact Sheet for TNT[6], d = EPA Technical Fact Sheet for RDX[7], f = EPA Technical Fact Sheet for DNT[8], g = EPA Technical Fact Sheet for Perchlorate[9], h = McGrath (1995)[10], i = Monteil-Rivera et al. (2004)[11], j = DNAN WEEL FINAL[12], l = DRDC 2011[13], m = NTO WEEL FINAL[14], n = van der Schalie (1985)[15], o = Hawari (2014)[16], p = Hawari et al. (2015)[17]. Table 2. Physical and chemical properties of important explosives and propellants. | |||||||||||
Health and Environmental Impacts
Exposure of humans to EM compounds can result in significant health issues. High oral or dermal exposures to TNT can cause liver and blood damage, anorexia, and anemia. High oral exposures to RDX can cause neurological affects such as convulsions. Some EM can bioaccumulate in crop plants (e.g. RDX), leading to potential exposure by eating or direct contact[18][19]. TNT and 2,4-DNT are classified as possible human carcinogens by the U.S. EPA[6]. In contrast, HMX is not currently classified as a human carcinogen, but has been shown to have adverse impacts on the liver and nervous system in some laboratory animals[20]. The US military is replacing many of traditional explosives with IMs to reduce risks of accidental detonation. Since IM materials including DNAN, NTO, and NQ have not been in common use, considerable effort has been focused on understanding the toxicity of these materials (see Munitions Constituents - IM Toxicology).
There are currently no federal maximum contaminant levels (MCLs) for TNT, RDX, and HMX. Life-time health advisory levels in drinking water vary from 400 µg/L for HMX, to 2 µg/L for RDX, and 2 µg/L for TNT[21].
Fate and Transport in the Environment
Much of the early work on environmental issues related to EM focused on concentrated sources including manufacturing facilities and locations where off-specification, unserviceable, and obsolete munitions were destroyed[22] . More recently, attention has focused on potential contamination from military training when partial detonations deposit EM particles on range soils (see Munitions Constituents - Deposition). Once deposited on a range, the EM dissolves over time, a process thought to be the rate-limiting step for aqueous transport of these compounds to soil and groundwater. The mass of EM dissolved is a function of the aqueous solubility of the compound, the mass of explosive residues deposited on the soil, the size of individual residues, and the three-dimensional (3D) structure of formulations that have multiple constituents (see Munitions Constituents - Dissolution). During transport through the subsurface, EM migration is influenced by sorption to the solid phase (see Munitions Constituents - Sorption), as well as chemical transformation and biodegradation.
However, the Department of Defense (DoD) has invested considerable resources to understand how these factors can be influenced, or how they operate in natural settings, to limit transport of EM in surface or groundwater. In addition, a number of remediation strategies have been developed that address EM, and applied both in situ and ex situ at DoD facilities.
References
- ^ 1.0 1.1 U.S. Environmental Protection Agency (USEPA), 2012. EPA Federal Facilities Forum Issue Paper: Site Characterization for Munitions Constituents, EPA/505/S-11/001, 2012. Report pdf
- ^ 2.0 2.1 Thiboutot, S., Ampleman, G. and Hewitt, A.D., 2002. Guide for characterization of sites contaminated with energetic materials (No. ERDC/CRREL-TR-02-1) U.S. Armu Environmental Center SFIM-AEC-TC-CR-200170. Report pdf
- ^ Jenkins, T.F., 2007. Energetic Munitions Constituents on DoD Training Ranges: Deposition, Accumulation, and Appropriate Characterization Technology, In: SERDP and ESTCP Technical Exchange Meeting on DoD Operational Range Assessment and Management Approaches, SERDP and ESTCP, Arlington, VA.
- ^ Fung, V., Schreiber, B., Patel, C., Samuels, P., Vinh, P. and Zhao, X.L., 2012. Process Improvement and Optimization of Insensitive Explosive IMX-101. In Insensitive Munitions & Munitions Constituents Technology Symposium (IMEMTS) & National Defense Industrial Association (NDIA): Las Vegas, NV, USA.
- ^ Pennington, J.C., Jenkins, T.F., Ampleman G., Thiboutot, S., Brannon, J.M., Hewitt, A.D., Lewis, J., Brochu, S., Diaz, E., Walsh, M.R., Walsh, M.E., Taylor, S., Lynch, J.C., Clausen, J., Ranney, T.A., Ramsey, C.A., Hayes, C.A., Grant, C.L., Collins, C.M., Bigl, S.R., Yost, S., Dontsova, K., 2006. Distribution and fate of energetics on DoD test and training ranges: Final Report. ERDC TR-06-13. Vicksburg, MS: U.S. Army Engineer Research and Development Center. Report pdf
- ^ 6.0 6.1 U.S. Environmental Protection Agency (USEPA), 2014. EPA Technical Fact Sheet - 2,4,6-Trinitrotoluene (TNT). Report pdf
- ^ U.S. Environmental Protection Agency (USEPA), 2014. EPA Technical Fact Sheet for RDX. Report pdf
- ^ U.S. Environmental Protection Agency (USEPA), 2014. EPA Technical Fact Sheet for DNT. Report pdf
- ^ U.S. Environmental Protection Agency (USEPA), 2014. EPA Technical Fact Sheet for Perchlorate. Report pdf
- ^ McGrath, C.J. 1995. Review of formulations for processes affecting the subsurface transport of explosives. IRRP-95-2, U.S. Army Engineer Waterways Experiment Station, Vicksburg, MS. Report pdf
- ^ Monteil-Rivera, F., Paquet, L., Deschamps, S., Balakrishnan, V.K., Beaulieu, C. and Hawari, J., 2004. Physico-chemical measurements of CL-20 for environmental applications: Comparison with RDX and HMX. Journal of Chromatography A, 1025(1), 125-132. doi: 10.1016/j.chroma.2003.08.060
- ^ OARS, 2014. Workplace environmental exposure level (WEEL) 2,4-Dinitroanisole (DNAN). OARS, Cincinnati, OH. Report pdf
- ^ DRDC, 2011. Annual Report 2010-2011. Environmental fate and ecological impact of emerging energetic chemicals (DNAN and its Amino-Derivatives, NTO, NQ, FOX-7, and FOX-12). Prepared by J. Hawari. NRC# 53363, Defense Research and Development Canada, National Research Council of Canada, Montréal, Québec.
- ^ OARS, 2014. Workplace environmental exposure level (WEEL) 3-Nitro-1,2,4-Triazol-5-One (NTO). OARS, Cincinnati, OH. Report pdf
- ^ Schalie, W.H., 1985. The toxicity of nitroguanidine and photolyzed nitroguanidine to freshwater aquatic organisms (No. USAMBRDL-TR-8404). Army Medical Bioengineering Research and Development Laboratory, Fort Detrick, MD. Report pdf
- ^ Hawari, J., 2014. Annual Report 2013-2014. Environmental fate and ecological impact of emerging energetic chemicals (ADN, DNAN and its Amino-Derivatives, PETN, NTO, NQ, FOX-7, and FOX-12) and an insensitive formulation. Defense Research and Development Canada, National Research Council of Canada, Montréal, Québec. Report pdf
- ^ Hawari, J., Monteil-Rivera, F., Perreault, N.N., Halasz, A., Paquet, L., Radovic-Hrapovic, Z., Deschamps, S., Thiboutot, S. and Ampleman, G., 2015. Environmental fate of 2, 4-dinitroanisole (DNAN) and its reduced products. Chemosphere, 119, 16-23. doi:10.1016/j.chemosphere.2014.05.047
- ^ Spain, J.C., Hughes, J.B. and Knackmuss, H.J. eds., 2000. Biodegradation of nitroaromatic compounds and explosives. CRC Press, 456 pgs.
- ^ Brannon, J.M. and Pennington, J.C., 2002. Environmental fate and transport process descriptors for explosives (No. ERDC/EL-TR-02-10). Engineer Research and development Center Vicksburg, MS Environmental Lab. Report pdf
- ^ Agency for Toxic Substances and Disease Registry (ATSDR), 1997. HMX Fact Sheet. Report pdf
- ^ United States Environmental Protection Agency (US EPA), 2009. Drinking water contaminant candidate list 3-final. Federal Register 74(194), 51850–51862. Report pdf
- ^ Spalding, R. and Fulton, J., 1988. Groundwater Munition Residues and Nitrate near Grand Island, Nebraska, USA. Journal of Contaminant Hydrology, 2(2), 139-153 doi:10.1016/0169-7722(88)90004-6
See Also
- Natural Attenuation of Explosives in Soil and Water Systems at DoD Sites
- A Predictive Capability for the Source Terms of Residual Munitions Constituents from Burning and/or Detonation Activities
- In-Situ Remediation of Explosives Contaminated Groundwater with Sequential Reactive Treatment Zones
- Bacterial Degradation of DNT and TNT Mixtures
- Microbial Degradation of RDX and HMX
- Novel Pathways of Nitroaromatic Metabolism: Hydroxylamine Formation, Reactivity and Potential for Ring Fission for Destruction of TNT
- Fe0-Based Bioremediation of RDX-Contaminated Groundwater
- Remediation of Explosives Contaminated Groundwater with Zero-Valent Iron
- Environmental Fate and Transport of a New Energetic Material, CL-20
- Environmental Fate and Transport of a New Energetic Material, CL-20
- Integrated Automated Analyzer for Monitoring of Explosives in Groundwater
- Long-Term Monitoring for Explosives-Contaminated Groundwater
- Enhancement of In Situ Bioremediation of Energetic Compounds by Coupled Abiotic/Biotic Processes
- Biodegradation of Nitroaromatic Compounds by Stimulating Humic Substance- and Fe(III)-Reduction
- Groundwater Chemistry and Microbial Ecology Effects on Explosives Biodegradation
- Development of Biomarkers for Assessing In Situ RDX Biodegradation Potential
- New Approaches to Evaluate the Biological Degradation of RDX in Groundwater
- Identification of Microbial Gene Biomarkers for In Situ RDX Biodegradation
- Monitored Natural Attenuation of Explosives in Groundwater
- Phytoremediation of Explosives-Contaminated Groundwater in Constructed Wetlands
- Portable SERS Instrument for Explosives Monitoring
- Biologically Active Zone Enhancement (BAZE) for In Situ RDX Degradation in Ground Water
- Electrically Induced Redox Barriers for In Situ Treatment of Groundwater
- Remediation of TNT and RDX in Groundwater Using Zero-Valent Iron Permeable Reactive Barriers and Zero-Valent Iron In Situ Treatment Wells
- In Situ Bioremediation of Energetic Compounds in Groundwater
- Treatment of RDX and HMX Plumes Using Mulch Biowalls
- Field Demonstration/Validation of Electrolytic Barriers for Energetic Compounds at Pueblo Chemical Depot
- Bioaugmentation for Aerobic Bioremediation of RDX-Contaminated Groundwater