Difference between revisions of "User:Jhurley/sandbox"
(→Environmental Fate) |
(→Transformation Processes) |
||
| Line 69: | Line 69: | ||
==Transformation Processes== | ==Transformation Processes== | ||
| + | Potential TCP degradation pathways include hydrolysis, oxidation, and reduction (Figure 2). These pathways are expected to be similar overall for abiotic and biotic reactions<ref name="Sarathy2010">Sarathy, V., Salter, A.J., Nurmi, J.T., O’Brien Johnson, G., Johnson, R.L., and Tratnyek, P.G., 2010. Degradation of 1, 2, 3-Trichloropropane (TCP): Hydrolysis, Elimination, and Reduction by Iron and Zinc. Environmental Science and Technology, 44(2), pp.787-793. [https://doi.org/10.1021/es902595j DOI: 10.1021/es902595j]</ref>, but the rates of the reactions (and their resulting significance for remediation) depend on natural and engineered conditions. | ||
| + | |||
| + | The rate of hydrolysis of TCP is negligible under typical ambient pH and temperature conditions but is favorable at high pH and/or temperature<ref name="Tratnyek2010"/><ref name="Sarathy2010"/>. For example, ammonia gas can be used to raise soil pH and stimulate alkaline hydrolysis of chlorinated propanes including TCP<ref name="Medina2016">Medina, V.F., Waisner, S.A., Griggs, C.S., Coyle, C., and Maxwell, M., 2016. Laboratory-Scale Demonstration Using Dilute Ammonia Gas-Induced Alkaline Hydrolysis of Soil Contaminants (Chlorinated Propanes and Explosives). US Army Engineer Research and Development Center, Environmental Laboratory (ERDC/EL), Report TR-16-10. [http://hdl.handle.net/11681/20312 Website] [[Media: ERDC_EL_TR_16_10.pdf | Report.pdf]]</ref>. [[Thermal Conduction Heating (TCH)]] may also produce favorable conditions for TCP hydrolysis<ref name="Tratnyek2010"/><ref name="Sarathy2010"/>. | ||
Revision as of 15:46, 7 October 2021
1,2,3-Trichloropropane (TCP)
1,2,3-Trichloropropane (TCP) is a chlorinated volatile organic compound (CVOC) that has been used in chemical production processes, in agriculture, and as a solvent, resulting in point and non-point source contamination of soil and groundwater. TCP is mobile and highly persistent in soil and groundwater. TCP is not currently regulated at the national level in the United States, but maximum contaminant levels (MCLs) have been developed by some states. Current treatment methods for TCP are limited and can be cost prohibitive. However, some treatment approaches, particularly in situ chemical reduction (ISCR) with zero valent zinc (ZVZ) and in situ bioremediation (ISB), have recently been shown to have potential as practical remedies for TCP contamination of groundwater.
Related Article(s):
Contributor(s):
- Alexandra J. Salter-Blanc
- Paul G. Tratnyek
- John Merrill
- Alyssa Saito
- Lea Kane
- Eric Suchomel
- Rula Deeb
Key Resource(s):
- Prospects for Remediation of 1,2,3-Trichloropropane by Natural and Engineered Abiotic Degradation Reactions. Strategic Environmental Research and Development Program (SERDP), Project ER-1457.[1]
- Verification Monitoring for In Situ Chemical Reduction Using Zero-Valent Zinc, A Novel Technology for Remediation of Chlorinated Alkanes. Strategic Environmental Research and Development Program (SERDP), Project ER-201628.[2]
Introduction
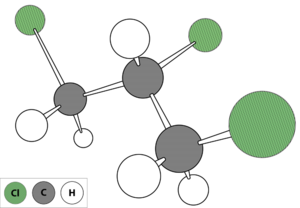
1,2,3-Trichloropropane (TCP) (Figure 1) is a man-made chemical that was used in the past primarily as a solvent and extractive agent, as a paint and varnish remover, and as a cleaning and degreasing agent.[3]. Currently, TCP is primarily used in chemical synthesis of compounds such as polysulfone liquid polymers used in the aerospace and automotive industries; hexafluoropropylene used in the agricultural, electronic, and pharmaceutical industries; polysulfide polymers used as sealants in manufacturing and construction; and 1,3-dichloropropene used in agriculture as a soil fumigant. TCP may also be present in products containing these chemicals as an impurity[3][4]. For example, the 1,2-dichlropropane/1,3-dichloropropene soil fumigant mixture (trade name D-D), which is no longer sold in the United States, contained TCP as an impurity and has been linked to TCP contamination in groundwater[5][4]. Soil fumigants currently in use which are composed primarily of 1,3-dichloropropene may also contain TCP as an impurity, for instance Telone II has been reported to contain up to 0.17 percent TCP by weight[6].
TCP contamination is problematic because it is “reasonably anticipated to be a human carcinogen” based on evidence of carcinogenicity to animals[7]. Toxicity to humans appears to be high relative to other chlorinated solvents[6], suggesting that even low-level exposure to TCP could pose a significant human health risk.
Environmental Fate
TCP’s fate in the environment is governed by its physical and chemical properties (Table 1). TCP does not adsorb strongly to soil, making it likely to leach into groundwater and exhibit high mobility. In addition, TCP is moderately volatile and can partition from surface water and moist soil into the atmosphere. Because TCP is only slightly soluble and denser than water, it can form a dense non-aqueous phase liquid (DNAPL) as observed at the Tyson’s Dump Superfund Site[8]. TCP is generally resistant to aerobic biodegradation, hydrolysis, oxidation, and reduction under naturally occurring conditions making it persistent in the environment[1].
| Property | Value |
|---|---|
| Chemical Abstracts Service (CAS) Number | 96-18-4 |
| Physical Description (at room temperature) |
Colorless to straw-colored liquid |
| Molecular weight (g/mol) |
147.43 |
| Water solubility at 25°C (mg/L) |
1,750 (slightly soluble) |
| Melting point (°C) |
-14.7 |
| Boiling point (°C) |
156.8 |
| Vapor pressure at 25°C (mm Hg) |
3.10 to 3.69 |
| Density at 20°C (g/cm3) | 1.3889 |
| Octanol-water partition coefficient (logKow) |
1.98 to 2.27 (temperature dependent) |
| Organic carbon-water partition coefficient (logKoc) |
1.70 to 1.99 (temperature dependent) |
| Henry’s Law constant at 25°C (atm-m3/mol) |
3.17x10-4[3] to 3.43x10-4[10] |
Occurrence
TCP has been detected in approximately 1% of public water supply and domestic well samples tested by the United States Geological Survey. More specifically, TCP was detected in 1.2% of public supply well samples collected between 1993 and 2007 by Toccalino and Hopple[11] and 0.66% of domestic supply well samples collected between 1991 and 2004 by DeSimone[12]. TCP was detected at a higher rate in domestic supply well samples associated with agricultural land-use studies than samples associated with studies comparing primary aquifers (3.5% versus 0.2%)[12].
Regulation
The United States Environmental Protection Agency (USEPA) has not established an MCL for TCP, although guidelines and health standards are in place[9]. TCP was included in the Contaminant Candidate List 3[13] and the Unregulated Contaminant Monitoring Rule 3 (UCMR 3)[14]. The UCMR 3 specified that data be collected on TCP occurrence in public water systems over the period of January 2013 through December 2015 against a reference concentration range of 0.0004 to 0.04 μg/L[15]. The reference concentration range was determined based on a cancer risk of 10-6 to 10-4 and derived from an oral slope factor of 30 mg/kg-day, which was determined by the EPA’s Integrated Risk Information System[16]. Of 36,848 samples collected during UCMR 3, 0.67% exceeded the minimum reporting level of 0.03 µg/L. 1.4% of public water systems had at least one detection over the minimum reporting level, corresponding to 2.5% of the population[15]. While these occurrence percentages are relatively low, the minimum reporting level of 0.03 µg/L is more than 75 times the USEPA-calculated Health Reference Level of 0.0004 µg/L. Because of this, TCP may occur in public water systems at concentrations that exceed the Health Reference Level but are below the minimum reporting level used during UCMR 3 data collection. These analytical limitations and lack of lower-level occurrence data have prevented the USEPA from making a preliminary regulatory determination for TCP[17].
Some US states have established their own standards including Hawaii which has established an MCL of 0.6 μg/L[18]. California has established an MCL of 0.005 μg/L[19], a notification level of 0.005 μg/L, and a public health goal of 0.0007 μg/L[20], and New Jersey has established an MCL of 0.03 μg/L[21].
Transformation Processes
Potential TCP degradation pathways include hydrolysis, oxidation, and reduction (Figure 2). These pathways are expected to be similar overall for abiotic and biotic reactions[22], but the rates of the reactions (and their resulting significance for remediation) depend on natural and engineered conditions.
The rate of hydrolysis of TCP is negligible under typical ambient pH and temperature conditions but is favorable at high pH and/or temperature[1][22]. For example, ammonia gas can be used to raise soil pH and stimulate alkaline hydrolysis of chlorinated propanes including TCP[23]. Thermal Conduction Heating (TCH) may also produce favorable conditions for TCP hydrolysis[1][22].
| Technology | Advantages | Limitations |
|---|---|---|
| ZVZ |
|
|
| Groundwater Extraction and Treatment |
|
|
| ZVI |
|
|
| ISCO |
|
|
| In Situ Bioremediation |
|
|
There are two main approaches to downscaling. One method, commonly referred to as “statistical downscaling”, uses the empirical-statistical relationships between large-scale weather phenomena and historical local weather data. In this method, these statistical relationships are applied to output generated by global climate models. A second method uses physics-based numerical models (regional-scale climate models or RCMs) of weather and climate that operate over a limited region of the earth (e.g., North America) and at spatial resolutions that are typically 3 to 10 times finer than the global-scale climate models. This method is known as “dynamical downscaling”. These regional-scale climate models are similar to the global models with respect to their reliance on the principles of physics, but because they operate over only part of the earth, they require information about what is coming in from the rest of the earth as well as what is going out of the limited region of the model. This is generally obtained from a global model. The primary differences between statistical and dynamical downscaling methods are summarized in Table 1.
It is important to realize that there is no “best” downscaling method or dataset, and that the best method/dataset for a given problem depends on that problem’s specific needs. Several data products based on downscaling higher level spatial data are available (USGS, MACA, NARCCAP, CORDEX-NA). The appropriate method and dataset to use depends on the intended application. The method selected should be able to credibly resolve spatial and temporal scales relevant for the application. For example, to develop a risk analysis of frequent flooding, the data product chosen should include precipitation at greater than a diurnal frequency and over multi-decadal timescales. This kind of product is most likely to be available using the dynamical downscaling method. SERDP reviewed the various advantages and disadvantages of using each type of downscaling method and downscaling dataset, and developed a recommended process that is publicly available[24]. In general, the following recommendations should be considered in order to pick the right downscaled dataset for a given analysis:
- When a problem depends on using a large number of climate models and emission scenarios to perform preliminary assessments and to understand the uncertainty range of projections, then using a statistical downscaled dataset is recommended.
- When the assessment needs a more extensive parameter list or is analyzing a region with few long-term observational data, dynamically downscaled climate change projections are recommended.
Uncertainty in Projections
| Model or Dataset Name |
Model Method |
Output Variables |
Output Format |
Spatial Resolution |
Time Resolution |
|---|---|---|---|---|---|
| Statistical Downscaled Datasets | |||||
| WorldClim[25] | Delta | T(min, max, avg), Pr |
NetCDF | grid: 30 arc sec to 10 arc min |
month |
| Bias Corrected / Spatial Disaggregation (BCSD)[26] |
Empirical Quantile Mapping |
Runoff, Streamflow |
NetCDF | grid: 7.5 arc min | day |
| Asynchronous Regional Regression Model (ARRM v.1)[27] |
Parameterized Quantile Mapping |
T(min, max), Pr | NetCDF | stations plus grid: 7.5 arc min |
day |
| Statistical Downscaling Model (SDSM)[28] | Weather Generator | T(min, max), Pr | PC Code | stations | day |
| Multivariate Adaptive Constructed Analogs (MACA)[29] |
Constructed Analogues | 10 Variables | NetCDF | grid: 2.5 arc min | day |
| Localized Constructed Analogs (LOCA)[30] | Constructed Analogues | T(min, max), Pr | NetCDF | grid: 3.75 arc min | day |
| NASA Earth Exchange Downscaled Climate Projections (NEX-DCP30)[26] |
Bias Correction / Spatial Disaggregation |
T(min, max), Pr | NetCDF | grid: 30 arc sec | month |
| Dynamical Downscaled Datasets | |||||
| North American Regional Climate Change Assessment Program (NARCCAP)[31] |
Multiple Models | 49 Variables | NetCDF | grid: 30 arc min | 3 hours |
| Coordinated Regional Climate Downscaling Experiment (CORDEX)[32] |
Multiple Models | 66 Variables | NetCDF | grid: 30 arc min | 3 hours |
| Strategic Environmental Research and Development Program (SERDP)[33] |
Weather Research and Forecasting (WRF v3.3) |
80+ Variables | NetCDF | grid: 6.5 arc min | 3 hours |
A primary cause of uncertainty in climate change projections, especially beyond 30 years into the future, is the uncertainty in the greenhouse gas (GHG) emission scenarios used to make climate model projections. The best method of accounting for this type of uncertainty is to apply a climate change model to multiple GHG emission scenarios (see also: Wikipedia: Representative Concentration Pathway).
The uncertainties in climate projections over shorter timescales, less than 30 years out, are dominated by something known as “internal variability” in the models. Different approaches are used to address the uncertainty from internal variability[34]. A third type of uncertainty in climate modeling, known as scientific uncertainty, comes from our inability to numerically solve every aspect of the complex earth system. We expect this scientific uncertainty to decrease as we understand more of the earth system and improve its representation in our numerical models. As discussed in Climate Change Primer, numerical experiments based on global climate models are designed to address these uncertainties in various ways. Downscaling methods evaluate this uncertainty by using several independent regional climate models to generate future projections, with the expectation that each of these models will capture some aspects of the physics better than the others, and that by using several different models, we can estimate the range of this uncertainty. Thus, the commonly accepted methods for accounting for uncertainty in climate model projections are either using projections from one model for several emission scenarios, or applying multiple models to project a single scenario.
A comparison of the currently available methods and their characteristics is provided in Table 2 (adapted from Kotamarthi et al., 2016[24]). The table lists the various methodologies and models used for producing downscaled data, and the climate variables that these methods produce. These datasets are mostly available for download from the data servers and websites listed in the table and in a few cases by contacting the respective source organizations.
The most popular and widely used format for atmospheric and climate science is known as NetCDF, which stands for Network Common Data Form. NetCDF is a self-describing data format that saves data in a binary format. The format is self-describing in that a metadata listing is part of every file that describes all the data attributes, such as dimensions, units and data size and in principal should not need additional information to extract the required data for analysis with the right software. However, specially built software for reading and extracting data from these binary files is necessary for making visualizations and further analysis. Software packages for reading and writing NetCDF datasets and for generating visualizations from these datasets are widely available and obtained free of cost (NetCDF-tools). Popular geospatial analysis tools such as ARC-GIS, statistical packages such as ‘R’ and programming languages such as Fortran, C++, and Python have built in libraries that can be used to directly read NetCDF files for visualization and analysis.
References
- ^ 1.0 1.1 1.2 1.3 Tratnyek, P.G., Sarathy, V., Salter, A.J., Nurmi, J.T., O’Brien Johnson, G., DeVoe, T., and Lee, P., 2010. Prospects for Remediation of 1,2,3-Trichloropropane by Natural and Engineered Abiotic Degradation Reactions. Strategic Environmental Research and Development Program (SERDP), Project ER-1457. Website Report.pdf
- ^ Kane, L.Z., Suchomel, E.J., and Deeb, R.A., 2020. Verification Monitoring for In Situ Chemical Reduction Using Zero-Valent Zinc, A Novel Technology for Remediation of Chlorinated Alkanes. Strategic Environmental Research and Development Program (SERDP), Project ER-201628. Website Report.pdf
- ^ 3.0 3.1 3.2 Agency for Toxic Substances and Disease Registry (ATSDR), 2021. Toxicological Profile for 1,2,3-Trichloropropane. Free download from: ATSDR Report.pdf
- ^ 4.0 4.1 CH2M HILL, 2005. Interim Guidance for Investigating Potential 1,2,3-Trichloropropane Sources in San Gabriel Valley Area 3. Report.pdf Website
- ^ Oki, D.S. and Giambelluca, T.W., 1987. DBCP, EDB, and TCP Contamination of Ground Water in Hawaii. Groundwater, 25(6), pp. 693-702. DOI: 10.1111/j.1745-6584.1987.tb02210.x
- ^ 6.0 6.1 Kielhorn, J., Könnecker, G., Pohlenz-Michel, C., Schmidt, S. and Mangelsdorf, I., 2003. Concise International Chemical Assessment Document 56: 1,2,3-Trichloropropane. World Health Organization, Geneva. Website Report.pdf
- ^ National Toxicology Program, 2016. Report on Carcinogens, 14th ed. U.S. Department of Health and Human Services, Public Health Service. Free download from: NIH Report.pdf
- ^ United States Environmental Protection Agency (USEPA), 2019. Fifth Five-year Review Report, Tyson’s Dump Superfund Site, Upper Merion Township, Montgomery County, Pennsylvania. Free download from: USEPA Report.pdf
- ^ 9.0 9.1 United States Environmental Protection Agency (USEPA), 2017. Technical Fact Sheet—1,2,3-Trichloropropane (TCP). EPA Project 505-F-17-007. 6 pp. Free download from: USEPA Report.pdf
- ^ Leighton Jr, D.T. and Calo, J.M., 1981. Distribution Coefficients of Chlorinated Hydrocarbons in Dilute Air-Water Systems for Groundwater Contamination Applications. Journal of Chemical and Engineering Data, 26(4), pp. 382-385. DOI: 10.1021/je00026a010
- ^ Toccalino, P.L., Norman, J.E., Hitt, K.J., 2010. Quality of Source Water from Public-Supply Wells in the United States, 1993–2007. Scientific Investigations Report 2010-5024. U.S. Geological Survey. DOI: 10.3133/sir20105024 Free download from: USGS Report.pdf
- ^ 12.0 12.1 DeSimone, L.A., 2009. Quality of Water from Domestic Wells in Principal Aquifers of the United States, 1991–2004. U.S. Geological Survey, Scientific Investigations Report 2008–5227. 139 pp. Free download from: USGS Report.pdf
- ^ United States Environmental Protection Agency (US EPA), 2009. Drinking Water Contaminant Candidate List 3-Final. Federal Register 74(194), pp. 51850–51862, Document E9-24287. Website Report.pdf
- ^ United States Environmental Protection Agency (US EPA), 2012. Revisions to the Unregulated Contaminant Mentoring Regulation (UCMR 3) for Public Water Systems. Federal Register 77(85) pp. 26072-26101. Website Report.pdf
- ^ 15.0 15.1 United States Environmental Protection Agency (USEPA), 2017. The Third Unregulated Contaminant Monitoring Rule (UCMR 3): Data Summary. EPA 815-S-17-001. Website Report.pdf
- ^ USEPA Integrated Risk Information System (IRIS), 2009. 1,2,3-Trichloropropane (CASRN 96-18-4). Website Summary.pdf
- ^ USEPA, 2021. Announcement of Final Regulatory Determinations for Contaminants on the Fourth Drinking Water Contaminant Candidate List. Free download from: USEPA Report.pdf
- ^ Hawaii Department of Health, 2013. Amendment and Compilation of Chapter 11-20 Hawaii Administrative Rules. Free download from: Hawaii Department of Health Report.pdf
- ^ California Code of Regulations, 2021. Section 64444 Maximum Contaminant Levels – Organic Chemicals (22 CA ADC § 64444). Website
- ^ Office of Environmental Health Hazard Assessment (OEHHA), California Environmental Protection Agency, 2009. Final Public Health Goal for 1,2,3-Trichloropropane in Drinking Water. Website
- ^ New Jersey Administrative Code 7:10, 2020. Safe Drinking Water Act Rules. Free download from: New Jersey Department of Environmental Protection
- ^ 22.0 22.1 22.2 Sarathy, V., Salter, A.J., Nurmi, J.T., O’Brien Johnson, G., Johnson, R.L., and Tratnyek, P.G., 2010. Degradation of 1, 2, 3-Trichloropropane (TCP): Hydrolysis, Elimination, and Reduction by Iron and Zinc. Environmental Science and Technology, 44(2), pp.787-793. DOI: 10.1021/es902595j
- ^ Medina, V.F., Waisner, S.A., Griggs, C.S., Coyle, C., and Maxwell, M., 2016. Laboratory-Scale Demonstration Using Dilute Ammonia Gas-Induced Alkaline Hydrolysis of Soil Contaminants (Chlorinated Propanes and Explosives). US Army Engineer Research and Development Center, Environmental Laboratory (ERDC/EL), Report TR-16-10. Website Report.pdf
- ^ 24.0 24.1 24.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedKotamarthi2016 - ^ Hijmans, R.J., Cameron, S.E., Parra, J.L., Jones, P.G. and Jarvis, A., 2005. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. International Journal of Climatology: A Journal of the Royal Meteorological Society, 25(15), pp 1965-1978. DOI: 10.1002/joc.1276
- ^ 26.0 26.1 Wood, A.W., Maurer, E.P., Kumar, A. and Lettenmaier, D.P., 2002. Long‐range experimental hydrologic forecasting for the eastern United States. Journal of Geophysical Research: Atmospheres, 107(D20), 4429, pp. ACL6 1-15. DOI:10.1029/2001JD000659 Free access article available from: American Geophysical Union Report.pdf
- ^ Stoner, A.M., Hayhoe, K., Yang, X., and Wuebbles, D.J., 2013. An Asynchronous Regional Regression Model for Statistical Downscaling of Daily Climate Variables. International Journal of Climatology, 33(11), pp. 2473-2494. DOI:10.1002/joc.3603
- ^ Wilby, R.L., and Dawson, C.W., 2013. The Statistical DownScaling Model: insights from one decade of application. International Journal of Climatology, 33(7), pp. 1707-1719. DOI: 10.1002/joc.3544
- ^ Hidalgo, H.G., Dettinger, M.D. and Cayan, D.R., 2008. Downscaling with Constructed Analogues: Daily Precipitation and Temperature Fields Over the United States. California Energy Commission PIER Final Project, Report CEC-500-2007-123. Report.pdf
- ^ Pierce, D.W., Cayan, D.R. and Thrasher, B.L., 2014. Statistical Downscaling Using Localized Constructed Analogs (LOCA). Journal of Hydrometeorology, 15(6), pp. 2558-2585. DOI: 10.1175/JHM-D-14-0082.1 Free access article available from: American Meteorological Society. Report.pdf
- ^ Mearns, L.O., Gutowski, W., Jones, R., Leung, R., McGinnis, S., Nunes, A. and Qian, Y., 2009. A Regional Climate Change Assessment Program for North America. Eos, Transactions, American Geophysical Union, 90(36), p.311. DOI: 10.1029/2009EO360002 Free access article from: American Geophysical Union Report.pdf
- ^ Giorgi, F., Jones, C., and Asrar, G.R., 2009. Addressing climate information needs at the regional level: the CORDEX framework. World Meteorological Organization (WMO) Bulletin, 58(3), pp. 175-183. Free access article from: World Meteorological Organization Report.pdf
- ^ Wang, J., and Kotamarthi, V.R., 2015. High‐resolution dynamically downscaled projections of precipitation in the mid and late 21st century over North America. Earth's Future, 3(7), pp. 268-288. DOI: 10.1002/2015EF000304 Free access article from: American Geophysical Union Report.pdf
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedKotamarthi2021
See Also
Climate Change Impacts to Department of Defense Installations, SERDP Project RC-2242