Supercritical Water Oxidation (SCWO)
Supercritical water oxidation (SCWO) is a single step wet oxidation process that transforms organic matter into water, carbon dioxide and, depending on the waste undergoing treatment, an inert mineral solid residue. The process is highly effective and can treat a variety of wet wastes without dewatering. The SCWO technology allows for the complete destruction of persistent and toxic organic contaminants such as perfluoroalkyl and polyfluoroalkyl substances (PFAS), 1,4-dioxane, and many more.
Related Article(s):
- Chlorinated Solvents
- Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)
- PFAS Ex Situ Water Treatment
- PFAS Sources
- PFAS Transport and Fate
- PFAS Treatment by Electrical Discharge Plasma
- Photoactivated Reductive Defluorination - PFAS Destruction
Contributor(s): Kobe Nagar and Dr. Marc A. Deshusses
Key Resource(s):
- Supercritical Water Oxidation as an Innovative Technology for PFAS Destruction[1].
- Treatment of municipal sewage sludge in supercritical water: A review[2].
- Supercritical Water Oxidation – Current Status of Full-scale Commercial Activity for Waste Destruction[3].
Introduction
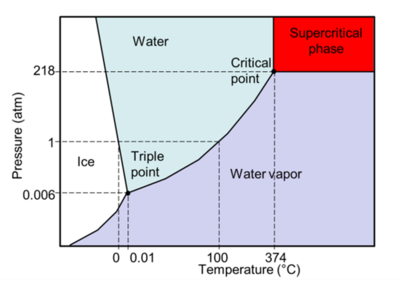
Supercritical water oxidation (SCWO) is an advanced oxidation process that holds enormous potential for the treatment of a wide range of organic wastes, in particular concentrated wet wastes in slurries such as biosolids, sludges, agricultural wastes, chemical wastes with recalcitrant chemicals such as perfluoroalkyl and polyfluoroalkyl substances (PFAS), and many more. SCWO relies on the unique reactivity and transport properties that occur when an aqueous waste stream is brought above the critical point of water (374°C and 218 atm, or 704°F and 3200 psi, see phase diagram in Figure 1). Supercritical water is a dense single phase with transport properties similar to those of a gas, and solvent properties comparable to those of a non-polar solvent[4]. Oxygen is fully soluble in supercritical water, resulting in extremely rapid and complete oxidation of all organics to carbon dioxide, clean water (that can be reused), and some non-leachable inorganic salts.
| Technology | SCWO | SCWG | HTL/HTC | WAO |
|---|---|---|---|---|
| Temperature | >380°C | >380°C | 250-300°C | 150-320°C |
| Pressure | >240 bar | >240 bar | 40-200 bar | 10-200 bar |
| Oxidant | Required | None | None | Required |
| Reaction time | 2-10 sec. | 40-90 sec. | 30 min. to 2 hr.s | 30 min. to 3 hr.s |
| Corrosion potential | Moderate to high | Moderate | Low | Low to moderate |
| Risk of reactor plugging | Moderate to high | High | High | Low |
| Reaction | Exothermic | Endothermic | Endothermic | Exothermic |
| Useable products | CO2 + clean H2O + heat + minerals |
Syngas (H2 + CH4 + CO) | Biocrude/Biochar | Possible H2O, volatile fatty acids |
| By-products | None | Tars, phenols, recalcitrant N, contaminated water |
Tars, phenols, recalcitrant N, contaminated water |
Tars, phenols, recalcitrant N, contaminated water |
| Fate of feedstock N, if any |
N2 gas | NH4+ in liquid effluent | NH4+ in liquid effluent + N in (by)-products |
NH4+ in liquid effluent + N in (by)-products |
| Notes: SCWG = supercritical water gasification, HTL/HTC = hydrothermal liquefaction/carbonization, WAO = wet air oxidation | ||||
For SCWO to be economical, the heat from the oxidation reaction is recovered and used in part to heat the influent stream, while the excess heat can be converted to electricity. Depending on the concentration of waste in the feedstock, SCWO reactors can be operated autothermally, i.e., no outside input of heat is required. Typical reaction times are in the order of 2-10 seconds, resulting in SCWO systems that are quite compact compared to other technologies (see Table 1). The process does not generate harmful by-products such as nitrogen oxides (NOx) or Sulfur oxides (SOx), carbon monoxide (CO), or odors[5]. Typically, if present, ammonia and organic nitrogen in the waste undergoing treatment are converted to nitrogen gas, while phosphorous precipitates as phosphates and can be recovered. When halogen containing contaminants are treated (e.g., PFAS), halogen-carbon bonds are generally broken and halide anions are released in solution (e.g., F- when treating PFAS or Cl- when treating trichloroethene (TCE) and tetrachloroethene (PCE)).
Advantages and Disadvantages
There are many advantages to SCWO treatment. SCWO is a destructive treatment in that the compounds treated are mineralized to simple elements or harmless molecules (e.g., water and carbon dioxide) rather than just being transferred to another medium. Another advantage is the absence of reaction by-products, incompletely oxidized contaminants or unreacted harmful oxidants (e.g., ozone). SCWO is an extremely rapid and effective reaction (typical reaction times are in the order of 5-10 seconds) making it possible to build systems that are very compact and have a high throughput. SCWO is also a very clean process. The highly oxidizing environment makes it possible to effectively treat all sorts of organic contaminants, often recalcitrant to other processes, with very high (>99%) destruction efficiencies. This includes treatment of trace contaminants, slurries of biosolids, waste oil, food wastes, plastics, or emerging contaminants such as PFAS or 1,4-dioxane. Also, the relatively moderate temperatures (380-600°C) compared to other destructive technologies such as incineration, combined with the presence of supercritical water prevent the formation of NOx and SOx compounds. Lastly, SCWO treatment does not require drying of the waste, and both liquids and slurries can be treated using SCWO.
There are several disadvantages to SCWO treatment. First, a significant amount of energy needs to be expended to bring the oxidant and the waste undergoing treatment to the critical point of water. Although a large fraction of this energy can be efficiently recovered in heat exchangers, compensating for heat losses constrains SCWO to the treatment of concentrated wastes with sufficient organic content for the exothermic oxidation reaction to provide the necessary heat. Typically, a minimum calorific content of around 2 MJ/kg (which generally corresponds to a chemical oxygen demand of about 120-150 g/L) is needed for autothermal operation. For more dilute streams, external heating or supplementation of fuel (diesel, alcohol, waste oil, etc.) can be implemented, but it can rapidly become cost prohibitive. Thus, SCWO is currently not economical for very large volumes (>50,000 gallon/day) of very dilute waste streams. A second limitation is related to the pumping of the waste. Because the process is conducted at high pressure (240 bars or 3500 psi), positive displacement pumps are required. This limits SCWO to liquids and slurries that can be pumped. Waste streams that contain excessive grit or abrasive materials, and soils cannot currently be processed using SCWO.
The many appealing benefits of supercritical water processing have stimulated engineers and entrepreneurs to invest significant efforts and resources in the development of the technology. Today, after roughly 30 years of development, commercial deployment is on the horizon[3]. Technical challenges that have slowed down commercial deployment of SCWO are linked to the complex nature of a high-pressure, high-temperature process. Critical issues include reactor materials selection to resist corrosion (typically high nickel alloys are used), reactor designs and construction to withstand the corrosive nature of the reactive mass, dealing with highly exothermic reactions at high pressure and high temperature, plugging of the reactor by minerals deposits, and energy recovery for autothermal operation. Another challenge was the unrealistic goal of some companies entering the SCWO market to produce power from waste streams (often wastewater sludge) at a competitive cost (3-5 cents/kWh). This was not feasible with the available technology, which led to several business failures.
The value proposition of treating recalcitrant wastes using SCWO is markedly different, especially in today’s context of increasing liability for trace levels of emerging contaminants such as PFAS. SCWO may prove to be the optimal treatment technology for many highly concentrated aqueous waste streams.
State of the Art
Relatively few large scale SCWO systems exist. Researchers at Duke University (Deshusses lab) have designed and built a prototype pilot-scale SCWO system housed in a standard 20-foot shipping container (Figure 2). This project was funded by the Reinvent the Toilet program of the Bill and Melinda Gates Foundation. The pilot system is a continuous process designed to treat 1 ton of sludge per day at 10-20% dry solids content. The unit has been undergoing testing at Duke since early 2015. The design includes moderate preheating of the waste slurry, followed by mixing with supercritical water (~600°C) and air, which serves as the oxidant. This internal mixing rapidly brings the waste undergoing treatment to supercritical conditions thereby minimizing corrosion and the risks of waste charring and associated reactor plugging. The organics in the sludge are rapidly oxidized to CO2, while the heat of oxidation is recovered to heat the influent waste. The reactor is a single tubular reactor. The high supercritical fluid velocity in the system helps with controlling mineral salts deposition in the reactor. The system is well instrumented, and operation is controlled using a supervisory control and data acquisition (SCADA) system with historian software for trends analysis and reporting of key performance indicators (e.g., temperatures and pressures, pollutant destruction). Experiments conducted with this pilot plant have shown effective treatment of a wide variety of otherwise problematic wastes such as primary, secondary and digested sludge slurries, landfill leachate (see Figure 3), animal waste, and co-contaminants including waste oil, food wastes, and plastics. The results are consistent with other SCWO studies and show very rapid treatment of all wastes with near complete conversion (often >99.9%) of organics to CO2. Total nitrogen and phosphorous removal are generally over 95% and 98%, respectively. Emerging contaminants such as pharmaceuticals, PFAS, 1,4-dioxane and microplastics are also treated with destruction generally exceeding 99%.
| Substance | Residual (ng/L) |
Removal |
|---|---|---|
| PFBA | 10.20 | 99.86% |
| PFHxA | 5.15 | 99.89% |
| PFNA | 1.07 | 99.90% |
| PFDA | 0.80 | 99.97% |
| PFUnA | <1.10 | >99.89% |
| PFBS | <0.19 | >99.98% |
| PFPes | <0.29 | >99.98% |
| PFHxS | 0.28 | 99.99% |
| PFOS | 0.65 | 99.99% |
| Note: Similar destruction efficiencies were obtained when treating AFFF solutions. | ||
Early projections for treatment costs (Capital Expenditures + Operating Expenditures) for slurries are in the range of $12 to $90 per ton (or $0.04 to $0.37 per gallon) depending on system scale and contaminant concentration, with a majority of the cost coming from amortizing the equipment. These cost projections make SCWO treatment very competitive compared to other treatment technologies for high-strength wastes. When treating large volumes of water, combining SCWO with another technology (e.g., nanofiltration, reverse osmosis, or adsorption onto GAC) should be considered so that only the concentrated brines or spent sorbent are treated using SCWO, thereby increasing the cost effectiveness of the overall treatment.
SCWO for the Treatment of PFAS and AFFF
Several reports have indicated that PFAS can be treated using SCWO[6]. Several runs treating biosolids known to contain PFAS as well as dilutions of pure aqueous film forming foam (AFFF) have also been conducted with the Duke SCWO system. Typical results are shown in Table 2. They indicate very effective treatment performance, with for example 110,000 ng/L PFOS in the feed reduced to 0.79 ng/L in the effluent, and many other PFAS reduced to below their detection limits. No HF was found in the effluent gas, and all the fluorine from the destroyed PFAS was accounted for as fluoride in the effluent water. These results show the ability of the SCWO process to destroy PFAS to levels well below the EPA health advisory levels of 70 ng/L for PFOS and PFOA. The Environmental Security Technology Certification Program (ESTCP) project number ER20-5350[7] launched in June 2020 will assess the technical feasibility of using supercritical water oxidation (SCWO) for the complete destruction of PFAS in a variety of relevant waste streams and will evaluate the cost effectiveness of the treatment. In parallel to the ESTCP demonstration, commercial deployment of the SCWO technology developed at Duke is conducted by 374Water, a social impact, cleantech company dedicated to the commercialization of supercritical water oxidation. 374Water offers modular SCWO systems for the treatment of a variety of wastes including biosolids and PFAS contaminated matrices.
References
- ^ Krause M. J., Thoma E.; Sahle-Damesessie E., Crone B., Whitehill A., Shields E., and Gullett B., 2022. Supercritical Water Oxidation as an Innovative Technology for PFAS Destruction. Journal of Environmental Engineering, 148 (2), 05021006. doi: 10.1061/(ASCE)EE.1943-7870.0001957
- ^ Qian, L., Wang, S., Xu, D., Guo, Y., Tang, X., and Wang, L., 2016. Treatment of municipal sewage sludge in supercritical water: A review. Water Research, 89, pp. 118-131. DOI: 10.1016/j.watres.2015.11.047 Free download from: ResearchGate
- ^ 3.0 3.1 Marrone, P.A., 2013. Supercritical Water Oxidation – Current Status of Full-scale Commercial Activity for Waste Destruction. Journal of Supercritical Fluids, 79, pp. 283-288. DOI: 10.1016/j.supflu.2012.12.020 Author’s manuscript available from: US EPA
- ^ Tassaing, T., Danten, Y., and Besnard, M., 2002. Infrared spectroscopic study of hydrogen bonding in water at high temperature and pressure. Journal of Molecular Liquids, 101(1-3), pp. 149-158. DOI: 10.1016/S0167-7322(02)00089-2
- ^ Bermejo, M.D. and Cocero, M.J., 2006. Supercritical water oxidation: A technical review. AIChE Journal, 52(11) pp. 3933-3951. DOI: 10.1002/aic.10993
- ^ Kucharzyk, K.H., Darlington, R., Benotti, M., Deeb, R. and Hawley, E., 2017. Novel treatment technologies for PFAS compounds: A critical review. Journal of Environmental Management, 204(2), pp. 757-764. DOI: 10.1016/j.jenvman.2017.08.016 Manuscript available from: ResearchGate
- ^ Deshusses, M.A., 2020. Supercritical Water Oxidation (SCWO) for Complete PFAS Destruction. Environmental Security Technology Certification Program (ESTCP) Project number ER20-5350. Project website