Difference between revisions of "Monitored Natural Attenuation (MNA) of Fuels"
m (1 revision imported) |
m (1 revision imported) |
(No difference)
| |
Revision as of 04:10, 4 May 2018
Monitored Natural Attenuation (MNA) is one of the most commonly used remediation approaches for groundwater contaminated with petroleum hydrocarbons (PHCs) and certain fuel additives such as fuel oxygenates or lead scavengers. Given appropriate conditions, MNA can be relied upon to attenuate concentrations of PHCs because they are very susceptible to many natural biodegradation processes and physical processes. Biodegradation reactions for hydrocarbons are ubiquitous at petroleum release sites, and most of the plumes are relatively short and either stable or shrinking. For fuel additives, MNA is used for plumes with low dissolved concentrations or in peripheral areas away from zones with nonaqueous phase liquids (NAPLs) or other materials that serve as the contamination source.
Related Article(s):
- Monitored Natural Attenuation (MNA)
- MNA of Chlorinated Solvents
- Biodegradation - Hydrocarbons
- Natural Source Zone Depletion (NSZD)
- Polycyclic Aromatic Hydrocarbons (PAHs)
CONTRIBUTOR(S): Dr. John Wilson
Key Resource(s):
Introduction
Fuel components, including PHCs such as toluene and fuel additives such as methyl tert-butyl ether (MTBE), are among the most abundant contaminants in groundwater. In 2006, the United States Geological Survey published results from a systematic survey of volatile organic chemicals in drinking water wells[2] in the U.S.A. Approximately 5% of samples from public wells contained MTBE at concentrations > 0.2 µg/L, and approximately 1% of wells contained toluene at concentrations > 0.2 µg/L.
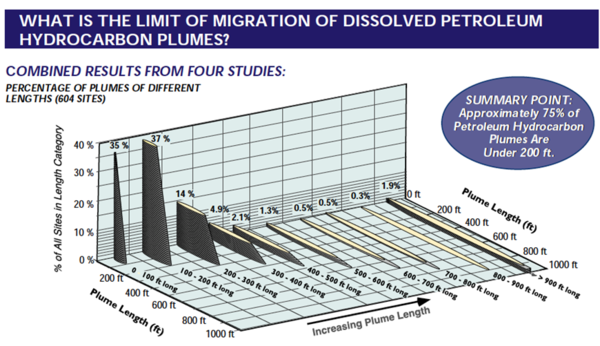
By far, the most common sources of fuel components and PHCs in groundwater are from underground storage tank (UST) fuel releases. Most fuel spills from UST sites produce plumes of contamination in groundwater that are relatively short. In a recent review[3] of available literature from the U.S.A, when the plume boundary is defined as 5 µg/L, the median plume length of benzene from UST spills was 180 feet, and the median length of MTBE plumes was 275 feet. Only 10% of benzene plumes were longer than 425 feet, and 10% of MTBE plumes were longer than 530 feet. For example, Figure 1 shows a compilation of four benzene plume length studies[1].
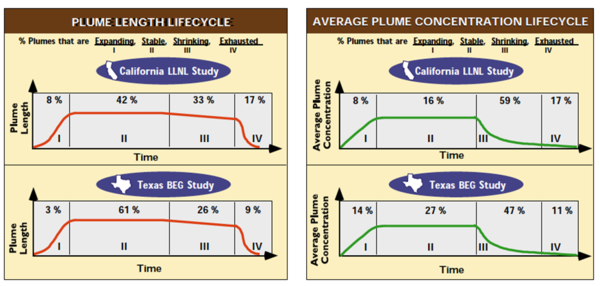
Plumes of petroleum contamination go through a 4-stage lifecycle (Fig. 2). 1) Shortly after the release, the concentrations in groundwater increase over time, and the plumes expand. 2) After some period of time, the plumes reach a stable configuration. After more time, the plumes start 3) to shrink, and eventually 4) the plumes are exhausted. The proportion of plumes that fall into the each of the four stages in the lifecycle is well documented in some states (Fig. 2).
Components of heavier fuel oils, such as complex polycyclic aromatic hydrocarbons (PAHs) like anthracene, phenanthrene and benzo[a]pyrene, more often occur from releases from aboveground storage tanks (ASTs) and oil terminal operations. These PHCs are far less soluble and do not form extensive plumes.
Regulatory Considerations
In the US, the primary responsibility for regulating releases from USTs falls to individual state agencies. Most states deal with fuel releases from USTs with some form of Risk-Based Corrective Action[4], which is based in full or in part on the ASTM International Standard Guide for Risk-Based Corrective Action, ASTM-E2081 (2015)[5]. Risk-Based Corrective Action is not MNA, however, Risk-Based Correction Action can rely on natural attenuation processes to manage the risk from fuel releases.
If a fuel spill occurs at a refinery, a distribution terminal, or a chemical manufacturing facility, it might be regulated under either the Resource Conservation and Recovery Act (RCRA), or the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA or Superfund).
MNA is one remedy that is available under RCRA or CERCLA. When regulated under RCRA, the usual goal is for the contaminants to attenuate to acceptable concentrations before groundwater can migrate off-site and impact receptors. Under this MNA approach, the groundwater must reach a cleanup goal before it reaches a point of compliance.
When regulated under CERCLA, there is often an additional requirement that all the contamination must reach the cleanup goal by a specified date. The performance of a remedy in Superfund is reviewed on a five-year cycle. A framework[8] is available to review long-term monitoring data to determine whether the attenuation within the review cycle is adequate to meet the cleanup goal by the specified date.
Degradation Process
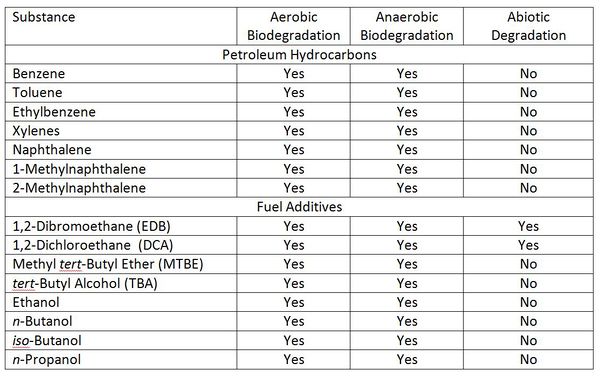
A number of natural processes can attenuate fuel components and PHCs in groundwater including biological degradation, abiotic degradation, sorption, dispersion into ground adjacent to the contaminant plume, and volatilization to soil gas above the groundwater (see also Table 1). At most sites where MNA has been selected as a remedy, or part of a remedy, these contaminants are degrading in groundwater. The geochemical conditions in the contaminated aquifer must be understood prior to proposing use of MNA of PHCs and fuel additives. These include at a minimum dissolved oxygen (DO), oxidation-reduction potential (ORP), nitrate, iron (ferric and ferrous), sulfate and alkalinity[9]. For example, analysis of 28 petroleum hydrocarbon sites by the US Air Force indicated that sulfate reduction and methanogenic degradation were the most important biodegradation processes at these sites, but acknowledged that degradation via iron reduction may be have been underestimated[10]. A long-term study of an oil spill showed that iron reduction degraded >10 times more oil than aerobic biodegradation[11].
Petroleum Hydrocarbons
PHCs of primary concern in groundwater are benzene, toluene, ethylbenzene, and the xylenes (the BTEX compounds) and naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene. These aromatic compounds are relatively soluble in water, and at fuel spill sites they often reach concentrations that exceed the standard that is allowed by U.S. EPA regulations[12] for contaminants in drinking water.
Primary aromatic compounds are readily degraded by bacteria if oxygen is available to support their metabolism. The rate of aerobic degradation is so rapid that by the time a sample of groundwater is acquired from a fuel spill, either the hydrocarbon is completely degraded and oxygen remains, or the oxygen is completely consumed and the hydrocarbon remains. The length of the contamination plume at a particular spill site is a function of the initial concentration of the petroleum hydrocarbon, the time required for biodegradation to attenuate the dissolved phase concentration to the appropriate standard, and the distance the groundwater moves in that time period.
Aromatic hydrocarbons such as benzene can be degraded in the absence of oxygen, but rates of degradation are much slower than degradation with oxygen. Instead of a half-life in the range of hours or days, the half-life will be in the range of months[13].
The biodegradation of PAHs is more difficult. Consequently, reliance on MNA to degrade PAHs in groundwater can be problematic. In one case, a detailed long-term mass balance of an oil spill in Minnesota revealed that oil constituents other than BTEX compounds, such as n-alkanes and aromatic compounds other than BTEX, play significant roles in source/plume natural attenuation[11]. Biodegradation processes were still on-going 30 years after the spill; and during this time an estimated 85-95% of the carbon biodegradation products were outgassed as methane or carbon dioxide.
Fuel Oxygenates (MTBE and TBA)
For many years, MTBE was added to gasoline to enhance the gasolines octane rating. In certain areas of the U.S.A, MTBE was added at higher concentrations to reduce air pollution. Early work suggested that MTBE was not biodegraded in groundwater, but later work showed that MTBE could be degraded[14] under aerobic[15] and anaerobic conditions[16]. The half-life for anaerobic degradation of MTBE in groundwater is roughly the same[16] as the half-life for anaerobic degradation of benzene.
A study of 48 MTBE plumes concluded that[17] “MTBE plumes in groundwater underlying a majority of these UST sites: i) have significantly diminished in concentration over time, ii) are comparable in length to benzene plumes, iii) are, like benzene plumes, principally stable or shrinking in size and concentration, and iv) are on track to achieve remedial goals within a timeframe comparable to or faster than that of benzene plumes” and that “natural attenuation is the principal mechanism of plume mass removal for” benzene, MTBE, and TBA. Another study of nine “exceptionally long “historic MTBE plumes demonstrated that five of the nine plumes decreased in length by 75% or more compared to their historical maximum lengths, and MTBE concentrations within these nine long plumes have decreased by 93% to 100%[18].
Degradation of MTBE can be recognized by a change in the ratios of stable isotopes of carbon and hydrogen in the MTBE remaining after degradation. These stable isotopes are said to be fractionated. Compound Specific Isotope Analysis (CSIA)[19] can provide unequivocal evidence that degradation has occurred in groundwater. However, MTBE can be degraded by different strains of bacteria using different mechanisms, and the extent of fractionation varies between the bacteria. Anaerobic degradation produces the strongest fractionation of carbon isotopes. As a result, the change in the ratio of carbon isotopes between MTBE at the source of contamination and MTBE in a plume in groundwater can be used to calculate a conservative lower boundary to the extent of degradation in the groundwater.
In addition to MTBE, gasoline often contained tert-butyl alcohol (TBA), which was present in the technical grade of MTBE. Biodegradation of MTBE under both aerobic and anaerobic conditions produces TBA (Fig. 3), and TBA accumulates in groundwater as MTBE degrades[20].
TBA is readily degraded when oxygen is available. It degrades more slowly under nitrate-reducing and sulfate-reducing conditions. However, there is little evidence that TBA is degradable under methanogenic conditions[14].
Lead Scavengers (EDB and DCA)
Leaded gasoline contained 1,2-dibromoethane (EDB) and 1,2-dichloroethane (DCA). These compounds prevented engine damage from the accumulation of lead in the engine. Although EDB was banned[21] in gasoline in the late 1980s, EDB is still present at many leaded gasoline spill sites[22]. Both EDB and DCA are degradable under aerobic and anaerobic conditions. The half-lives for anaerobic degradation of EDB and DCA are in the same range as the half-life for anaerobic degradation of benzene. Both EDB and DCA are degraded through an abiotic reaction with iron monosulfide (FeS)[22] that can be produced in sulfate-reducing aquifers.
Fuel Alcohols
Ethanol, n-butanol, iso-butanol, and n-propanol are added to gasoline to enhance the fuel's octane rating. They also function as a fuel oxygenate to reduce air pollution from combustion of the fuel. These alcohols are readily biodegradable in groundwater under aerobic and anaerobic conditions. The degradation of ethanol can create conditions that inhibit the degradation of the BTEX compounds[23], and enhances the degradation of MTBE to TBA[24].
Summary
MNA is one of the most commonly used remediation approaches for petroleum hydrocarbon plumes. Aerobic and anaerobic biodegradation reactions for hydrocarbons are ubiquitous at petroleum release sites, and most hydrocarbon plumes are relatively short and either stable or shrinking. MNA processes are also active for MTBE plumes and other fuel additives.
References
- ^ 1.0 1.1 1.2 1.3 Newell, C.J., Connor, J.A., 1998. Characteristics of dissolved petroleum hydrocarbon plumes, results from four studies. American Petroleum Institute. Washington, D.C. Report pdf
- ^ Zogorski, J.S., Carter, J.M., Ivahnenko, T., Lapham, W.W., Moran, M.J., Rowe, B.L., Squillace, P.J., Toccalino, P.L., 2006. Volatile organic compounds in the nation’s ground water and drinking-water supply wells. US Geological Survey Circular, 1292, 101. Report pdf
- ^ Connor, J.A., Kamath, R., Walker, K.L., McHugh, T.E., 2014. Review of quantitative surveys of the length and stability of MTBE, TBA, and benzene plumes in groundwater at UST sites. Groundwater, 53, 195–206. doi: 10.1111/gwat.12233
- ^ Introduction to Risk-Based Corrective Action (RBCA). Michigan Department of Environmental Quality. DEQ RBCA
- ^ ASTM, 2015. Standard Guide for Risk Based Corrective Action. ASTM E2081 - 00(2015). Standard Guide for RBCA
- ^ Rice, D.W., Grose, R.D., Michaelsen, J.C., Dooher, B.P., MacQueen, D.H., Cullen, S.J., Kastenberg, W.E., Everett, L.G., Marino, M.A., 1995. California leaking underground fuel tank (LUFT) historical case analysis. Environmental Protection Department. Report pdf
- ^ Mace, R.E., Fisher, R.S., Welch, D.M., Parra, S.P., 1997. Extent, mass, and duration of hydrocarbon plumes from leaking petroleum storage tank sites in Texas. Bureau of Economic Geology, University of Texas at Austin. Geologic Circular 97-1. Geologic Circular GC9701
- ^ Wilson, J.T., 2011. An Approach for Evaluating the Progress of Natural Attenuation in Groundwater. EPA 600-R-11-204. Report pdf
- ^ Wiedemeier, T.H., Wilson, J.T., Kampbell, D.H., Miller, R.N., Hansen, J.E., 1999. Technical protocol for implementing intrinsic remediation with long-term monitoring for natural attenuation of fuel contamination dissolved in groundwater. Volume I. Report pdf
- ^ Newell, C.J., Gonzales, J., McLeod, R., 1996. BIOSCREEN natural attenuation decision support system, U.S. Environmental Protection Agency. EPA/600/R-96/087. Report pdf
- ^ 11.0 11.1 Ng, G.H.C., Bekins, B.A., Cozzarelli, I.M., Baedecker, M.J., Bennett, P.C., Amos, R.T., 2014. A mass balance approach to investigating geochemical controls on secondary water quality impacts at a crude oil spill site near Bemidji, MN. Journal of Contaminant Hydrology, 164, 1-15. doi: 10.1016/j.jconhyd.2014.04.006
- ^ U.S. Environmental Protection Agency, 2016. Table of Regulated Drinking Water Contaminants. Table of Regulated Drinking Water
- ^ Suarez, M.P., Rifai, H.S., 1999. Biodegradation rates for fuel hydrocarbons and chlorinated solvents in groundwater. Bioremediation Journal, 3(4), 337-362. doi: 10.1080/10889869991219433
- ^ 14.0 14.1 Schmidt, T.C., Schirmer, M., Weiß, H., Haderlein, S.B., 2004. Microbial degradation of methyl tert-butyl ether and tert-butyl alcohol in the subsurface. Journal of Contaminant Hydrology, 70(3), 173-203. doi:10.1016/j.jconhyd.2003.09.001
- ^ Deeb, R.A., Scow, K.M., Alvarez-Cohen, L., 2000. Aerobic MTBE biodegradation: an examination of past studies, current challenges and future research directions. Biodegradation, 11(2-3), 171-185. doi: 10.1023/a:1011113320414
- ^ 16.0 16.1 Wilson, J.T., Kaiser, P.M., Adair, C., 2005. Monitored natural attenuation of MTBE as a risk management option at leaking underground storage tank sites EPA/600/R-04/1790. Report pdf
- ^ Kamath, R., Adamson, D.T. , Newell, C.J., Vangelas, K.M., and Looney, B.B., 2010. Passive soil vapor extraction. Savannah River National Laboratory, Aiken, South Carolina. SRNL-STI-2009-00571, Rev. 1. Report pdf
- ^ McDade, J.M., Connor, J.A., Paquette, S.M., Small, J.M., 2015. Exceptionally long MTBE plumes of the past have greatly diminished. Groundwater, 53(4), 515-524. doi: 10.1111/gwat.12322
- ^ Hunkeler, D., Meckenstock, R. U., Sherwood Lollar, B., Schmidt, T. C., Wilson, J. T., 2008. A Guide for Assessing Biodegradation and Source Identification of Organic Groundwater Contaminants Using Compound Specific Isotope Analysis (CSIA). U.S. Environmental Protection Agency, Washington, D.C., EPA/600/R-08/148. Report pdf
- ^ McHugh, T.E., Kulkarni, P.R., Newell, C.J., Connor, J.A., Garg, S., 2014. Progress in remediation of groundwater at petroleum sites in California. Groundwater, 52(6), 898-907. doi: 10.1111/gwat.12136
- ^ Falta, R.W., 2005. The Potential for Ground Water Contamination by the Gasoline Lead Scavengers Ethylene Dibromide and 1, 2‐Dichloroethane. Environmental Science and Technology Magazine Online. Report pdf
- ^ 22.0 22.1 Wilson, J.T., Banks, K., Earle, R.C., He, Y., Kuder, T., Adair, C., 2008. Natural attenuation of the lead scavengers 1, 2-dibromoethane (EDB) and 1, 2-dichloroethane (1, 2-DCA) at motor fuel release sites and implications for risk management. US Environmental Protection Agency, Office of Research and Development, National Risk Management Research Laboratory. Report pdf
- ^ Corseuil, H.X., Monier, A.L., Fernandes, M., Schneider, M.R., Nunes, C.C., do Rosario, M., Alvarez, P.J., 2011. BTEX plume dynamics following an ethanol blend release: geochemical footprint and thermodynamic constraints on natural attenuation. Environmental Science & Technology, 45(8), 3422-3429. doi: 10.1021/es104055q
- ^ Mackay, D., de Sieyes, N., Einarson, M., Feris, K., Pappas, A., Wood, I., Jacobson, L., Justice, L., Noske, M., Wilson, J., Adair, C., 2007. Impact of ethanol on the natural attenuation of MTBE in a normally sulfate-reducing aquifer. Environmental Science & Technology, 41(6), 2015-2021. doi:10.1021/es062156q