Difference between revisions of "User:Jhurley/sandbox"
(→Fiber-optic Distributed Temperature Sensing (FO-DTS)) |
(→Fiber-optic Distributed Temperature Sensing (FO-DTS)) |
||
| Line 48: | Line 48: | ||
=====Fiber-optic Distributed Temperature Sensing (FO-DTS)===== | =====Fiber-optic Distributed Temperature Sensing (FO-DTS)===== | ||
| − | Fiber-optic distributed temperature sensing (FO-DTS) is a powerful monitoring technology used in fire detection, industrial process monitoring, and petroleum reservoir monitoring. The method is also used to obtain spatially rich datasets for monitoring GWSWE<ref name=”Selker2006”>Selker, J. S., Thévenaz, L., Huwald, H., Mallet, A., Luxemburg, W., van de Giesen, N., Stejskal, M., Zeman, J., Westhoff, M., Parlange, M. B., 2006. Distributed Fiber-Optic Temperature Sensing for Hydrologic Systems. Water Resources Research, 42 (12). [https://doi.org/10.1029/2006WR005326 doi: 10.1029/2006WR005326]. [https://agupubs.onlinelibrary.wiley.com/doi/epdf/10.1029/2006WR005326 Open Access Article]</ref><ref name=”Tyler2009”>Tyler, S. W., Selker, J. S., Hausner, M. B., Hatch, C. E., Torgersen, T., Thodal, C. E., Schladow, S. G., 2009. Environmental Temperature Sensing Using Raman Spectra DTS Fiber-Optic Methods. Water Resources Research, 45(4). [https://doi.org/https://doi.org/10.1029/2008WR007052 doi: 10.1029/2008WR007052]. [https://agupubs.onlinelibrary.wiley.com/doi/epdf/10.1029/2008WR007052 Open Access Article]</ref>. The sensor consists of standard telecommunications fiber-optic fiber typically housed in armored cable and the physics is based on temperature-dependent backscatter mechanisms including Brillouin and Raman backscatter< | + | Fiber-optic distributed temperature sensing (FO-DTS) is a powerful monitoring technology used in fire detection, industrial process monitoring, and petroleum reservoir monitoring. The method is also used to obtain spatially rich datasets for monitoring GWSWE<ref name=”Selker2006”>Selker, J. S., Thévenaz, L., Huwald, H., Mallet, A., Luxemburg, W., van de Giesen, N., Stejskal, M., Zeman, J., Westhoff, M., Parlange, M. B., 2006. Distributed Fiber-Optic Temperature Sensing for Hydrologic Systems. Water Resources Research, 42 (12). [https://doi.org/10.1029/2006WR005326 doi: 10.1029/2006WR005326]. [https://agupubs.onlinelibrary.wiley.com/doi/epdf/10.1029/2006WR005326 Open Access Article]</ref><ref name=”Tyler2009”>Tyler, S. W., Selker, J. S., Hausner, M. B., Hatch, C. E., Torgersen, T., Thodal, C. E., Schladow, S. G., 2009. Environmental Temperature Sensing Using Raman Spectra DTS Fiber-Optic Methods. Water Resources Research, 45(4). [https://doi.org/https://doi.org/10.1029/2008WR007052 doi: 10.1029/2008WR007052]. [https://agupubs.onlinelibrary.wiley.com/doi/epdf/10.1029/2008WR007052 Open Access Article]</ref>. The sensor consists of standard telecommunications fiber-optic fiber typically housed in armored cable and the physics is based on temperature-dependent backscatter mechanisms including Brillouin and Raman backscatter<ref name=”Selker2006”/>. |
Revision as of 09:48, 25 January 2024
Hydrogeophysical methods for characterization and monitoring of surface water-groundwater interactions
Hydrogeophysical methods can be used to cost-effectively locate and characterize regions of enhanced groundwater/surface-water exchange (GWSWE) and to guide effective follow up investigations based on more traditional invasive methods. The most established methods exploit the contrasts in temperature and/or specific conductance that commonly exist between groundwater and surface water.
Related Article(s):
Contributor(s):
- Dr. Lee Slater
- Dr. Ramona Iery
- Dr. Dimitrios Ntarlagiannis
- Henry Moore
Key Resource(s):
- USGS Method Selection Tool: https://code.usgs.gov/water/espd/hgb/gw-sw-mst
- USGS Water Resources: https://www.usgs.gov/mission-areas/water-resources/science/groundwatersurface-water-interaction
Introduction
Discharges of contaminated groundwater to surface water bodies threaten ecosystems and degrade the quality of surface water resources. Subsurface heterogeneity associated with the geological setting and stratigraphy often results in such discharges occurring as localized zones (or seeps) of contaminated groundwater. Traditional methods for investigating GWSWE include seepage meters[1][2], which directly quantify the volume flux crossing the bed of a surface water body (i.e, a lake, river or wetland) and point probes that locally measure key water quality parameters (e.g., temperature, pore water velocity, specific conductance, dissolved oxygen, pH). Seepage meters provide direct estimates of seepage fluxes between groundwater and surface- water but are time consuming and can be difficult to deploy in high energy surface water environments and along armored bed sediments. Manual seepage meters rely on quantifying volume changes in a bag of water that is hydraulically connected to the bed. Although automated seepage meters such as the Ultraseep system have been developed, they are generally not suitable for long term deployment (weeks to months). The US Navy has developed the Trident probe for more rapid (relative to seepage meters) sampling, whereby the probe is inserted into the bed and point-in-time pore water quality and sediment parameters are directly recorded (note that the Trident probe does not measure a seepage flux). Such direct probe-based measurements are still relatively time consuming to acquire, particularly when reconnaissance information is required over large areas to determine the location of discrete seeps for further, more quantitative analysis.
Over the last few decades, a broader toolbox of hydrogeophysical technologies has been developed to rapidly and non-invasively evaluate zones of GWSWE in a variety of surface water settings, spanning from freshwater bodies to saline coastal environments. Many of these technologies are currently being deployed under a Department of Defense Environmental Security Technology Certification Program (ESTCP) project (ER21-5237) to demonstrate the value of the toolbox to remedial program managers (RPMs) dealing with the challenge of characterizing surface water contamination via groundwater from facilities proximal to surface water bodies. This article summarizes these technologies and provides references to key resources, mostly provided by the Water Resources Mission Area of the United States Geological Survey that describe the technologies in further detail.
Hydrogeophysical Technologies for Understanding Groundwater-Surface Water Interactions
Hydrogeophysical technologies exploit contrasts in the physical properties between groundwater and surface water to detect and monitor zones of pronounced GWSWE. The two most valuable properties to measure are temperature and electrical conductivity. Temperature has been used for decades as an indicator of groundwater-surface water exchange[3] with early uses including pushing a thermistor into the bed of a surface water body to assess zones of surface water downwelling and groundwater upwelling. Today, a variety of novel technologies that measure temperature over a wide range of spatial and temporal scales are being used to investigate GWSWE. The evaluation of electrical conductivity measurements using point probes and geophysical imaging is also well-established. However, new technologies are now available to exploit electrical conductivity contrasts from GWSWE occurring over a range of spatial and temporal scales.
Temperature-Based Technologies
Several temperature-based GWSWE methodologies exploit the gradient in temperature between surface water and groundwater that exist during certain times of day or seasons of the year. The thermal insulation provided by the Earth’s land surface means that groundwater is warmer than surface water in winter months, but colder than surface water in summer months away from the equator. Therefore, in temperate climates, localized (or ‘preferential’) groundwater discharge into surface water bodies is often observed as cold temperature anomalies in the summer and warm temperature anomalies in the winter. However, there are times of the year such as fall and spring when contrasts in the temperature between groundwater and surface water will be minimal, or even undetectable. These seasonal-driven points in time correspond to the switch in the polarity of the temperature contrast between groundwater and surface water. Consequently, SW to GW gradient temperature-based methods are most effective when deployed at times of the year when the temperature contrasts between groundwater and surface water are greatest. Other time-series temperature monitoring methods depend more on natural daily signals measured at the bed interface and in bed sediments, and those signals may exist year round except where strongly muted by ice cover or surface water stratification. A variety of sensing technologies now exist within the GWSWE toolbox, from techniques that rapidly characterize temperature contrasts over large areas, down to powerful monitoring methods that can continuously quantify GWSWE fluxes at discrete locations identified as hotspots.
Characterization Methods
The primary use of the characterization methods is to rapidly determine precise locations of groundwater upwelling over large areas in order to pinpoint locations for subsequent ground-based observations. A common limitation of these methods is that they can only sense groundwater fluxes into surface water. Methods applied at the water surface and in the surface water column generally cannot detect localized regions of surface water transfer to groundwater, for which temperature measurements collected within the bed sediments are needed. This is a more challenging characterization task that may, in the right conditions, be addressed using electrical conductivity-based methods described later in this article.
Unmanned Aerial Vehicle Infrared (UAV-IR)
Unmanned aerial vehicles (UAVs) equipped with thermal infrared (IR) cameras can provide a very powerful tool for rapidly determining zones of pronounced upwelling of groundwater to surface water. Large areas of can be covered with high spatial resolution. The information obtained can be used to rapidly define locations of focused groundwater upwelling and prioritize these for more intensive surface-based observations (Figure 1). As with all thermal methods, flights must be performed when adequate contrasts in temperature between surface water and groundwater are expected to exist. Not just time of year but, because of the effect of the diurnal temperature signal on surface water bodies, time of day might need to be considered in order to maximize the chance of success. Calibration of UAV-IR camera measurements against simultaneously acquired direct measurements of temperature is recommended to optimize the value of these datasets. UAV-IR methods will not work in all situations. One major limitation of the technology is that the temperature expression of groundwater upwelling must be manifested at the surface of the surface water body. Consequently, the technology will not detect relatively small discharges occurring beneath a relatively deep surface water layer, and thermal imaging over the water surface can be complicated by thermal IR reflection. The chances of success with UAV-IR will be strongest in regions of exposed banks or shallow water where there are no strong currents causing mixing (and thus dilution) of the upwelling groundwater temperature signals. UAV-IR methods will therefore likely be most successful close to shorelines of lakes/ponds, over shallow, low flow streams and rivers and in wetland environments. UAV-IR methods require a licensed pilot, and restrictions on the use of airspace may limit the application of this technology.
Handheld Thermal Infrared (TIR) Cameras
Hand-held thermal infrared (TIR) cameras are powerful tools for visual identification of localized seeps of upwelling groundwater. TIR cameras may be used to follow up on UAV-IR surveys to better characterize local seeps identified from the air using UAV-IR. Alternatively, a TIR camera is a valuable tool when performing initial walks of prospective study sites as they may quickly confirm the presence of suspected seeps. TIR cameras provide high resolution images that can define the structure of localized seeps and may provide valuable insights into the role of discrete features (e.g., fractures in rocks or pipes in soil) in determining seep morphology (Figure 2). Like UAV-IR, TIR provides primarily qualitative information (location, extent) of seeps and it only succeeds when there are adequate contrasts between groundwater and surface water that are expressed at the surface of the investigated water body or along bank sediments. The United States Geological Survey (USGS) has made extensive use of TIR cameras for studying groundwater/surface-water exchange.
Continuous Near-bed Temperature Sensing
When performing surveys from a boat a simple yet often powerful technology is continuous near-bed temperature sensing, whereby a temperature probe is strategically suspended to float in the water column just above the bed or dragged along it. Compared to UAV-IR, this approach does not rely on upwelling groundwater being expressed as a temperature anomaly at the surface. The utility of the method can be enhanced when a specific conductance probe is co- located with the temperature probe so that anomalies in both temperature and specific conductance can be investigated.
Monitoring Methods
Monitoring methods allow temperature signals to be recorded with high temporal resolution along the bed interface or within bank or bed sediments. These methods can capture temporal trends in GWSWE driven by variations in the hydraulic gradients around surface water bodies, as well as changes in hydraulic conductivity due to sedimentation, clogging, scour or microbial mass. If vertical profiles of bed temperature are collected, a range of analytical and numerical models can be applied to infer the vertical water flux rate and direction, similar to a seepage meter. These fluxes may vary as a function of season, rainfall events (enhanced during storm activity), tidal variability in coastal settings and due to engineered controls such as dam discharges. The methods can capture evidence of GWSWE that may not be detected during a single ‘characterization’ survey if the local hydraulic conditions at that point in time result in relatively weak hydraulic gradients.
Fiber-optic Distributed Temperature Sensing (FO-DTS)
Fiber-optic distributed temperature sensing (FO-DTS) is a powerful monitoring technology used in fire detection, industrial process monitoring, and petroleum reservoir monitoring. The method is also used to obtain spatially rich datasets for monitoring GWSWE[7][8]. The sensor consists of standard telecommunications fiber-optic fiber typically housed in armored cable and the physics is based on temperature-dependent backscatter mechanisms including Brillouin and Raman backscatter[7].
The two most predominant forms of organic carbon in natural systems are natural organic matter (NOM) and black carbon (BC)[9]. Black carbon includes charcoal, soot, graphite, and coal. Until the early 2000s black carbon was considered to be a class of (bio)chemically inert geosorbents[10]. However, it has been shown that BC can contain abundant quinone functional groups and thus can store and exchange electrons[11] with chemical[12] and biological[13] agents in the surroundings. Specifically, BC such as biochar has been shown to reductively transform MCs including NTO, DNAN, and RDX[14].
NOM encompasses all the organic compounds present in terrestrial and aquatic environments and can be classified into two groups, non-humic and humic substances. Humic substances (HS) contain a wide array of functional groups including carboxyl, enol, ether, ketone, ester, amide, (hydro)quinone, and phenol[15]. Quinone and hydroquinone groups are believed to be the predominant redox moieties responsible for the capacity of HS and BC to store and reversibly accept and donate electrons (i.e., through reduction and oxidation of HS/BC, respectively)[16][17][11][18][19][20][21][22][23][24][25][26][27][28][29].
Hydroquinones have been widely used as surrogates to understand the reductive transformation of NACs and MCs by NOM. Figure 4 shows the chemical structures of the singly deprotonated forms of four hydroquinone species previously used to study NAC/MC reduction. The second-order rate constants (kR) for the reduction of NACs/MCs by these hydroquinone species are listed in Table 1, along with the aqueous-phase one electron reduction potentials of the NACs/MCs (EH1’) where available. EH1’ is an experimentally measurable thermodynamic property that reflects the propensity of a given NAC/MC to accept an electron in water (EH1(R-NO2)):
- Equation 1: R-NO2 + e- ⇔ R-NO2•-
Knowing the identity of and reaction order in the reductant is required to derive the second-order rate constants listed in Table 1. This same reason limits the utility of reduction rate constants measured with complex carbonaceous reductants such as NOM[17], BC[30][31][32][33], and HS[34][35], whose chemical structures and redox moieties responsible for the reduction, as well as their abundance, are not clearly defined or known. In other words, the observed rate constants in those studies are specific to the experimental conditions (e.g., pH and NOM source and concentration), and may not be easily comparable to other studies.
| Compound | EH1' (V) | Hydroquinone [log kR (M-1s-1)] | |||
|---|---|---|---|---|---|
| (NAC/MC) | LAW- | JUG- | AHQDS- | AHQS- | |
| Nitrobenzene (NB) | -0.485[16] | 0.380[16] | -1.102[16] | 2.050[36] | 3.060[36] |
| 2-nitrotoluene (2-NT) | -0.590[16] | -1.432[16] | -2.523[16] | 0.775[37] | |
| 3-nitrotoluene (3-NT) | -0.475[16] | 0.462[16] | -0.921[16] | ||
| 4-nitrotoluene (4-NT) | -0.500[16] | 0.041[16] | -1.292[16] | 1.822[37] | 2.610[36] |
| 2-chloronitrobenzene (2-ClNB) | -0.485[16] | 0.342[16] | -0.824[16] | 2.412[37] | |
| 3-chloronitrobenzene (3-ClNB) | -0.405[16] | 1.491[16] | 0.114[16] | ||
| 4-chloronitrobenzene (4-ClNB) | -0.450[16] | 1.041[16] | -0.301[16] | 2.988[37] | |
| 2-acetylnitrobenzene (2-AcNB) | -0.470[16] | 0.519[16] | -0.456[16] | ||
| 3-acetylnitrobenzene (3-AcNB) | -0.405[16] | 1.663[16] | 0.398[16] | ||
| 4-acetylnitrobenzene (4-AcNB) | -0.360[16] | 2.519[16] | 1.477[16] | ||
| 2-nitrophenol (2-NP) | 0.568 (0.079)[16] | ||||
| 4-nitrophenol (4-NP) | -0.699 (-1.301)[16] | ||||
| 4-methyl-2-nitrophenol (4-Me-2-NP) | 0.748 (0.176)[16] | ||||
| 4-chloro-2-nitrophenol (4-Cl-2-NP) | 1.602 (1.114)[16] | ||||
| 5-fluoro-2-nitrophenol (5-Cl-2-NP) | 0.447 (-0.155)[16] | ||||
| 2,4,6-trinitrotoluene (TNT) | -0.280[38] | 2.869[39] | 5.204[37] | ||
| 2-amino-4,6-dinitrotoluene (2-A-4,6-DNT) | -0.400[38] | 0.987[39] | |||
| 4-amino-2,6-dinitrotoluene (4-A-2,6-DNT) | -0.440[38] | 0.079[39] | |||
| 2,4-diamino-6-nitrotoluene (2,4-DA-6-NT) | -0.505[38] | -1.678[39] | |||
| 2,6-diamino-4-nitrotoluene (2,6-DA-4-NT) | -0.495[38] | -1.252[39] | |||
| 1,3-dinitrobenzene (1,3-DNB) | -0.345[39] | 1.785[39] | |||
| 1,4-dinitrobenzene (1,4-DNB) | -0.257[39] | 3.839[39] | |||
| 2-nitroaniline (2-NANE) | < -0.560[39] | -2.638[39] | |||
| 3-nitroaniline (3-NANE) | -0.500[39] | -1.367[39] | |||
| 1,2-dinitrobenzene (1,2-DNB) | -0.290[39] | 5.407[37] | |||
| 4-nitroanisole (4-NAN) | -0.661[36] | 1.220[36] | |||
| 2-amino-4-nitroanisole (2-A-4-NAN) | -0.924[36] | 1.150[36] | 2.190[36] | ||
| 4-amino-2-nitroanisole (4-A-2-NAN) | 1.610[36] | 2.360[36] | |||
| 2-chloro-4-nitroaniline (2-Cl-5-NANE) | -0.863[36] | 1.250[36] | 2.210[36] | ||
| N-methyl-4-nitroaniline (MNA) | -1.740[36] | -0.260[36] | 0.692[36] | ||
| 3-nitro-1,2,4-triazol-5-one (NTO) | 5.701 (1.914)[35] | ||||
| Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) | -0.349[40] | ||||
Most of the current knowledge about MC degradation is derived from studies using NACs. The reduction kinetics of only four MCs, namely TNT, N-methyl-4-nitroaniline (MNA), NTO, and RDX, have been investigated with hydroquinones. Of these four MCs, only the reduction rates of MNA and TNT have been modeled[39][36][41][42].
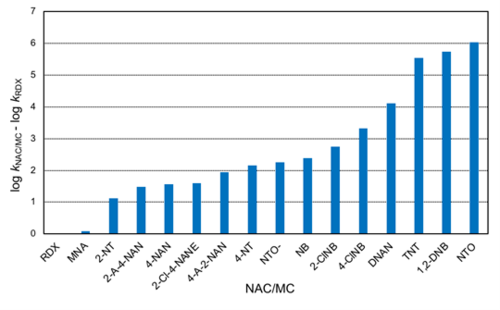
Using the rate constants obtained with AHQDS–, a relative reactivity trend can be obtained (Figure 5). RDX is the slowest reacting MC in Table 1, hence it was selected to calculate the relative rates of reaction (i.e., log kNAC/MC – log kRDX). If only the MCs in Figure 5 are considered, the reactivity spans 6 orders of magnitude following the trend: RDX ≈ MNA < NTO– < DNAN < TNT < NTO. The rate constant for DNAN reduction by AHQDS– is not yet published and hence not included in Table 1. Note that speciation of NACs/MCs can significantly affect their reduction rates. Upon deprotonation, the NAC/MC becomes negatively charged and less reactive as an oxidant (i.e., less prone to accept an electron). As a result, the second-order rate constant can decrease by 0.5-0.6 log unit in the case of nitrophenols and approximately 4 log units in the case of NTO (numbers in parentheses in Table 1)[16][35].
Ferruginous Reductants
| Compound | EH1' (V) | Cysteine[43] [FeL2]2- |
Thioglycolic acid[43] [FeL2]2- |
DFOB[44] [FeHL]0 |
AcHA[44] [FeL3]- |
Tiron a [FeL2]6- |
Fe-Porphyrin b |
|---|---|---|---|---|---|---|---|
| Fe(II)-Ligand [log kR (M-1s-1)] | |||||||
| Nitrobenzene | -0.485[16] | -0.347 | 0.874 | 2.235 | -0.136 | 1.424[45] 4.000[42] |
-0.018[16] 0.026[42] |
| 2-nitrotoluene | -0.590[16] | -0.602[16] | |||||
| 3-nitrotoluene | -0.475[16] | -0.434 | 0.767 | 2.106 | -0.229 | 1.999[45] 3.800[42] |
0.041[16] |
| 4-nitrotoluene | -0.500[16] | -0.652 | 0.528 | 2.013 | -0.402 | 1.446[45] 3.500[42] |
-0.174[16] |
| 2-chloronitrobenzene | -0.485[16] | 0.944[16] | |||||
| 3-chloronitrobenzene | -0.405[16] | 0.360 | 1.810 | 2.888 | 0.691 | 2.882[45] 4.900[42] |
0.724[16] |
| 4-chloronitrobenzene | -0.450[16] | 0.230 | 1.415 | 2.512 | 0.375 | 3.937[45] 4.581[46] |
0.431[16] 0.289[42] |
| 2-acetylnitrobenzene | -0.470[16] | 1.377[16] | |||||
| 3-acetylnitrobenzene | -0.405[16] | 0.799[16] | |||||
| 4-acetylnitrobenzene | -0.360[16] | 0.965 | 2.771 | 1.872 | 5.028[45] 6.300[42] |
1.693[16] | |
| RDX | -0.550[47] | 2.212[45] 2.864[48] |
|||||
| HMX | -0.660[47] | -2.762[45] | |||||
| TNT | -0.280[38] | 7.427[45] | 2.050[42] | ||||
| 1,3-dinitrobenzene | -0.345[39] | 1.220[42] | |||||
| 2,4-dinitrotoluene | -0.380[38] | 5.319[45] | 1.156[42] | ||||
| Nitroguanidine (NQ) | -0.700[47] | -0.185[45] | |||||
| 2,4-dinitroanisole (DNAN) | -0.400[47] | 1.243[42] | |||||
| Notes: a 4,5-dihydroxybenzene-1,3-disulfonate (Tiron). b meso-tetra(N-methyl-pyridyl)iron porphin in cysteine. | |||||||
| MC | Iron Mineral | Iron mineral loading (g/L) |
Surface area (m2/g) |
Fe(II)aq initial (mM) b |
Fe(II)aq after 24 h (mM) c |
Fe(II)aq sorbed (mM) d |
pH | Buffer | Buffer (mM) |
MC initial (μM) e |
log kobs (h-1) f |
log kSA (Lh-1m-2) g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TNT[39] | Goethite | 0.64 | 17.5 | 1.5 | 7.0 | MOPS | 25 | 50 | 1.200 | 0.170 | ||
| RDX[49] | Magnetite | 1.00 | 44 | 0.1 | 0 | 0.10 | 7.0 | HEPES | 50 | 50 | -3.500 | -5.200 |
| RDX[49] | Magnetite | 1.00 | 44 | 0.2 | 0.02 | 0.18 | 7.0 | HEPES | 50 | 50 | -2.900 | -4.500 |
| RDX[49] | Magnetite | 1.00 | 44 | 0.5 | 0.23 | 0.27 | 7.0 | HEPES | 50 | 50 | -1.900 | -3.600 |
| RDX[49] | Magnetite | 1.00 | 44 | 1.5 | 0.94 | 0.56 | 7.0 | HEPES | 50 | 50 | -1.400 | -3.100 |
| RDX[49] | Magnetite | 1.00 | 44 | 3.0 | 1.74 | 1.26 | 7.0 | HEPES | 50 | 50 | -1.200 | -2.900 |
| RDX[49] | Magnetite | 1.00 | 44 | 5.0 | 3.38 | 1.62 | 7.0 | HEPES | 50 | 50 | -1.100 | -2.800 |
| RDX[49] | Magnetite | 1.00 | 44 | 10.0 | 7.77 | 2.23 | 7.0 | HEPES | 50 | 50 | -1.000 | -2.600 |
| RDX[49] | Magnetite | 1.00 | 44 | 1.6 | 1.42 | 0.16 | 6.0 | MES | 50 | 50 | -2.700 | -4.300 |
| RDX[49] | Magnetite | 1.00 | 44 | 1.6 | 1.34 | 0.24 | 6.5 | MOPS | 50 | 50 | -1.800 | -3.400 |
| RDX[49] | Magnetite | 1.00 | 44 | 1.6 | 1.21 | 0.37 | 7.0 | MOPS | 50 | 50 | -1.200 | -2.900 |
| RDX[49] | Magnetite | 1.00 | 44 | 1.6 | 1.01 | 0.57 | 7.0 | HEPES | 50 | 50 | -1.200 | -2.800 |
| RDX[49] | Magnetite | 1.00 | 44 | 1.6 | 0.76 | 0.82 | 7.5 | HEPES | 50 | 50 | -0.490 | -2.100 |
| RDX[49] | Magnetite | 1.00 | 44 | 1.6 | 0.56 | 1.01 | 8.0 | HEPES | 50 | 50 | -0.590 | -2.200 |
| NG[50] | Magnetite | 4.00 | 0.56 | 4.0 | 7.4 | HEPES | 90 | 226 | ||||
| NG[51] | Pyrite | 20.00 | 0.53 | 7.4 | HEPES | 100 | 307 | -2.213 | -3.238 | |||
| TNT[51] | Pyrite | 20.00 | 0.53 | 7.4 | HEPES | 100 | 242 | -2.812 | -3.837 | |||
| RDX[51] | Pyrite | 20.00 | 0.53 | 7.4 | HEPES | 100 | 201 | -3.058 | -4.083 | |||
| RDX[52] | Carbonate Green Rust | 5.00 | 36 | 7.0 | 100 | |||||||
| RDX[52] | Sulfate Green Rust | 5.00 | 20 | 7.0 | 100 | |||||||
| DNAN[53] | Sulfate Green Rust | 10.00 | 8.4 | 500 | ||||||||
| NTO[53] | Sulfate Green Rust | 10.00 | 8.4 | 500 | ||||||||
| DNAN[54] | Magnetite | 2.00 | 17.8 | 1.0 | 7.0 | NaHCO3 | 10 | 200 | -0.100 | -1.700 | ||
| DNAN[54] | Mackinawite | 1.50 | 7.0 | NaHCO3 | 10 | 200 | 0.061 | |||||
| DNAN[54] | Goethite | 1.00 | 103.8 | 1.0 | 7.0 | NaHCO3 | 10 | 200 | 0.410 | -1.600 | ||
| RDX[55] | Magnetite | 0.62 | 1.0 | 7.0 | NaHCO3 | 10 | 17.5 | -1.100 | ||||
| RDX[55] | Magnetite | 0.62 | 7.0 | MOPS | 50 | 17.5 | -0.270 | |||||
| RDX[55] | Magnetite | 0.62 | 1.0 | 7.0 | MOPS | 10 | 17.6 | -0.480 | ||||
| NTO[56] | Hematite | 1.00 | 5.7 | 1.0 | 0.92 | 0.08 | 5.5 | MES | 50 | 30 | -0.550 | -1.308 |
| NTO[56] | Hematite | 1.00 | 5.7 | 1.0 | 0.85 | 0.15 | 6.0 | MES | 50 | 30 | 0.619 | -0.140 |
| NTO[56] | Hematite | 1.00 | 5.7 | 1.0 | 0.9 | 0.10 | 6.5 | MES | 50 | 30 | 1.348 | 0.590 |
| NTO[56] | Hematite | 1.00 | 5.7 | 1.0 | 0.77 | 0.23 | 7.0 | MOPS | 50 | 30 | 2.167 | 1.408 |
| NTO[56] | Hematite a | 1.00 | 5.7 | 1.01 | 5.5 | MES | 50 | 30 | -1.444 | -2.200 | ||
| NTO[56] | Hematite a | 1.00 | 5.7 | 0.97 | 6.0 | MES | 50 | 30 | -0.658 | -1.413 | ||
| NTO[56] | Hematite a | 1.00 | 5.7 | 0.87 | 6.5 | MES | 50 | 30 | 0.068 | -0.688 | ||
| NTO[56] | Hematite a | 1.00 | 5.7 | 0.79 | 7.0 | MOPS | 50 | 30 | 1.210 | 0.456 | ||
| RDX[57] | Mackinawite | 0.45 | 6.5 | NaHCO3 | 10 | 250 | -0.092 | |||||
| RDX[57] | Mackinawite | 0.45 | 7.0 | NaHCO3 | 10 | 250 | 0.009 | |||||
| RDX[57] | Mackinawite | 0.45 | 7.5 | NaHCO3 | 10 | 250 | 0.158 | |||||
| RDX[57] | Green Rust | 5 | 6.5 | NaHCO3 | 10 | 250 | -1.301 | |||||
| RDX[57] | Green Rust | 5 | 7.0 | NaHCO3 | 10 | 250 | -1.097 | |||||
| RDX[57] | Green Rust | 5 | 7.5 | NaHCO3 | 10 | 250 | -0.745 | |||||
| RDX[57] | Goethite | 0.5 | 1 | 1 | 6.5 | NaHCO3 | 10 | 250 | -0.921 | |||
| RDX[57] | Goethite | 0.5 | 1 | 1 | 7.0 | NaHCO3 | 10 | 250 | -0.347 | |||
| RDX[57] | Goethite | 0.5 | 1 | 1 | 7.5 | NaHCO3 | 10 | 250 | 0.009 | |||
| RDX[57] | Hematite | 0.5 | 1 | 1 | 6.5 | NaHCO3 | 10 | 250 | -0.824 | |||
| RDX[57] | Hematite | 0.5 | 1 | 1 | 7.0 | NaHCO3 | 10 | 250 | -0.456 | |||
| RDX[57] | Hematite | 0.5 | 1 | 1 | 7.5 | NaHCO3 | 10 | 250 | -0.237 | |||
| RDX[57] | Magnetite | 2 | 1 | 1 | 6.5 | NaHCO3 | 10 | 250 | -1.523 | |||
| RDX[57] | Magnetite | 2 | 1 | 1 | 7.0 | NaHCO3 | 10 | 250 | -0.824 | |||
| RDX[57] | Magnetite | 2 | 1 | 1 | 7.5 | NaHCO3 | 10 | 250 | -0.229 | |||
| DNAN[58] | Mackinawite | 4.28 | 0.25 | 6.5 | NaHCO3 | 8.5 + 20% CO2(g) | 400 | 0.836 | 0.806 | |||
| DNAN[58] | Mackinawite | 4.28 | 0.25 | 7.6 | NaHCO3 | 95.2 + 20% CO2(g) | 400 | 0.762 | 0.732 | |||
| DNAN[58] | Commercial FeS | 5.00 | 0.214 | 6.5 | NaHCO3 | 8.5 + 20% CO2(g) | 400 | 0.477 | 0.447 | |||
| DNAN[58] | Commercial FeS | 5.00 | 0.214 | 7.6 | NaHCO3 | 95.2 + 20% CO2(g) | 400 | 0.745 | 0.716 | |||
| NTO[58] | Mackinawite | 4.28 | 0.25 | 6.5 | NaHCO3 | 8.5 + 20% CO2(g) | 1000 | 0.663 | 0.633 | |||
| NTO[58] | Mackinawite | 4.28 | 0.25 | 7.6 | NaHCO3 | 95.2 + 20% CO2(g) | 1000 | 0.521 | 0.491 | |||
| NTO[58] | Commercial FeS | 5.00 | 0.214 | 6.5 | NaHCO3 | 8.5 + 20% CO2(g) | 1000 | 0.492 | 0.462 | |||
| NTO[58] | Commercial FeS | 5.00 | 0.214 | 7.6 | NaHCO3 | 95.2 + 20% CO2(g) | 1000 | 0.427 | 0.398 | |||
| Notes: a Dithionite-reduced hematite; experiments conducted in the presence of 1 mM sulfite. b Initial aqueous Fe(II); not added for Fe(II) bearing minerals. c Aqueous Fe(II) after 24h of equilibration. d Difference between b and c. e Initial nominal MC concentration. f Pseudo-first order rate constant. g Surface area normalized rate constant calculated as kObs / (surface area concentration) or kObs / (surface area × mineral loading). | ||||||||||||
| NAC a | Iron Oxide | Iron oxide loading (g/L) |
Surface area (m2/g) |
Fe(II)aq initial (mM) b |
Fe(II)aq after 24 h (mM) c |
Fe(II)aq sorbed (mM) d |
pH | Buffer | Buffer (mM) |
NAC initial (μM) e |
log kobs (h-1) f |
log kSA (Lh-1m-2) g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NB[59] | Magnetite | 0.200 | 56.00 | 1.5000 | 7.00 | Phosphate | 10 | 50 | 1.05E+00 | 7.75E-04 | ||
| 4-ClNB[59] | Magnetite | 0.200 | 56.00 | 1.5000 | 7.00 | Phosphate | 10 | 50 | 1.14E+00 | 8.69E-02 | ||
| 4-ClNB[39] | Goethite | 0.640 | 17.50 | 1.5000 | 7.00 | MOPS | 25 | 50 | -1.01E-01 | -1.15E+00 | ||
| 4-ClNB[60] | Goethite | 1.500 | 16.20 | 1.2400 | 0.9600 | 0.2800 | 7.20 | MOPS | 1.2 | 0.5 - 3 | 1.68E+00 | 2.80E-01 |
| 4-ClNB[60] | Hematite | 1.800 | 13.70 | 1.0400 | 1.0100 | 0.0300 | 7.20 | MOPS | 1.2 | 0.5 - 3 | -2.32E+00 | -3.72E+00 |
| 4-ClNB[60] | Lepidocrocite | 1.400 | 17.60 | 1.1400 | 1.0000 | 0.1400 | 7.20 | MOPS | 1.2 | 0.5 - 3 | 1.51E+00 | 1.20E-01 |
| 4-CNNB[61] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3500 | 0.0300 | 7.97 | HEPES | 25 | 15 | -7.47E-01 | -8.61E-01 |
| 4-CNNB[61] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3700 | 0.0079 | 7.67 | HEPES | 25 | 15 | -1.51E+00 | -1.62E+00 |
| 4-CNNB[61] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3600 | 0.0200 | 7.50 | MOPS | 25 | 15 | -2.15E+00 | -2.26E+00 |
| 4-CNNB[61] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3600 | 0.0120 | 7.28 | MOPS | 25 | 15 | -3.08E+00 | -3.19E+00 |
| 4-CNNB[61] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3700 | 0.0004 | 7.00 | MOPS | 25 | 15 | -3.22E+00 | -3.34E+00 |
| 4-CNNB[61] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3700 | 0.0024 | 6.80 | MOPSO | 25 | 15 | -3.72E+00 | -3.83E+00 |
| 4-CNNB[61] | Ferrihydrite | 0.004 | 292.00 | 0.3750 | 0.3700 | 0.0031 | 6.60 | MES | 25 | 15 | -3.83E+00 | -3.94E+00 |
| 4-CNNB[61] | Ferrihydrite | 0.020 | 292.00 | 0.3750 | 0.3700 | 0.0031 | 6.60 | MES | 25 | 15 | -3.83E+00 | -4.60E+00 |
| 4-CNNB[61] | Ferrihydrite | 0.110 | 292.00 | 0.3750 | 0.3700 | 0.0032 | 6.60 | MES | 25 | 15 | -1.57E+00 | -3.08E+00 |
| 4-CNNB[61] | Ferrihydrite | 0.220 | 292.00 | 0.3750 | 0.3700 | 0.0040 | 6.60 | MES | 25 | 15 | -1.12E+00 | -2.93E+00 |
| 4-CNNB[61] | Ferrihydrite | 0.551 | 292.00 | 0.3750 | 0.3700 | 0.0092 | 6.60 | MES | 25 | 15 | -6.18E-01 | -2.82E+00 |
| 4-CNNB[61] | Ferrihydrite | 1.099 | 292.00 | 0.3750 | 0.3500 | 0.0240 | 6.60 | MES | 25 | 15 | -3.66E-01 | -2.87E+00 |
| 4-CNNB[61] | Ferrihydrite | 1.651 | 292.00 | 0.3750 | 0.3400 | 0.0340 | 6.60 | MES | 25 | 15 | -8.35E-02 | -2.77E+00 |
| 4-CNNB[61] | Ferrihydrite | 2.199 | 292.00 | 0.3750 | 0.3300 | 0.0430 | 6.60 | MES | 25 | 15 | -3.11E-02 | -2.84E+00 |
| 4-CNNB[61] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3320 | 0.0430 | 7.97 | HEPES | 25 | 15 | 1.63E+00 | 1.52E+00 |
| 4-CNNB[61] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3480 | 0.0270 | 7.67 | HEPES | 25 | 15 | 1.26E+00 | 1.15E+00 |
| 4-CNNB[61] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3470 | 0.0280 | 7.50 | MOPS | 25 | 15 | 7.23E-01 | 6.10E-01 |
| 4-CNNB[61] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3680 | 0.0066 | 7.28 | MOPS | 25 | 15 | 4.53E-02 | -6.86E-02 |
| 4-CNNB[61] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3710 | 0.0043 | 7.00 | MOPS | 25 | 15 | -3.12E-01 | -4.26E-01 |
| 4-CNNB[61] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3710 | 0.0042 | 6.80 | MOPSO | 25 | 15 | -7.75E-01 | -8.89E-01 |
| 4-CNNB[61] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3680 | 0.0069 | 6.60 | MES | 25 | 15 | -1.39E+00 | -1.50E+00 |
| 4-CNNB[61] | Hematite | 0.038 | 34.00 | 0.3750 | 0.3750 | 0.0003 | 6.10 | MES | 25 | 15 | -2.77E+00 | -2.88E+00 |
| 4-CNNB[61] | Hematite | 0.016 | 34.00 | 0.3750 | 0.3730 | 0.0024 | 6.60 | MES | 25 | 15 | -3.20E+00 | -2.95E+00 |
| 4-CNNB[61] | Hematite | 0.024 | 34.00 | 0.3750 | 0.3690 | 0.0064 | 6.60 | MES | 25 | 15 | -2.74E+00 | -2.66E+00 |
| 4-CNNB[61] | Hematite | 0.033 | 34.00 | 0.3750 | 0.3680 | 0.0069 | 6.60 | MES | 25 | 15 | -1.39E+00 | -1.43E+00 |
| 4-CNNB[61] | Hematite | 0.177 | 34.00 | 0.3750 | 0.3640 | 0.0110 | 6.60 | MES | 25 | 15 | 3.58E-01 | -4.22E-01 |
| 4-CNNB[61] | Hematite | 0.353 | 34.00 | 0.3750 | 0.3630 | 0.0120 | 6.60 | MES | 25 | 15 | 9.97E-01 | -8.27E-02 |
| 4-CNNB[61] | Hematite | 0.885 | 34.00 | 0.3750 | 0.3480 | 0.0270 | 6.60 | MES | 25 | 15 | 1.34E+00 | -1.34E-01 |
| 4-CNNB[61] | Hematite | 1.771 | 34.00 | 0.3750 | 0.3380 | 0.0370 | 6.60 | MES | 25 | 15 | 1.78E+00 | 3.59E-03 |
| 4-CNNB[61] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3460 | 0.0290 | 7.97 | HEPES | 25 | 15 | 1.31E+00 | 1.20E+00 |
| 4-CNNB[61] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3610 | 0.0140 | 7.67 | HEPES | 25 | 15 | 5.82E-01 | 4.68E-01 |
| 4-CNNB[61] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3480 | 0.0270 | 7.50 | MOPS | 25 | 15 | 4.92E-02 | -6.47E-02 |
| 4-CNNB[61] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3640 | 0.0110 | 7.28 | MOPS | 25 | 15 | 1.62E+00 | -4.90E-01 |
| 4-CNNB[61] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3640 | 0.0110 | 7.00 | MOPS | 25 | 15 | -1.25E+00 | -1.36E+00 |
| 4-CNNB[61] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3620 | 0.0130 | 6.80 | MOPSO | 25 | 15 | -1.74E+00 | -1.86E+00 |
| 4-CNNB[61] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3740 | 0.0015 | 6.60 | MES | 25 | 15 | -2.58E+00 | -2.69E+00 |
| 4-CNNB[61] | Lepidocrocite | 0.027 | 49.00 | 0.3750 | 0.3700 | 0.0046 | 6.10 | MES | 25 | 15 | -3.80E+00 | -3.92E+00 |
| 4-CNNB[61] | Lepidocrocite | 0.020 | 49.00 | 0.3750 | 0.3740 | 0.0014 | 6.60 | MES | 25 | 15 | -2.58E+00 | -2.57E+00 |
| 4-CNNB[61] | Lepidocrocite | 11.980 | 49.00 | 0.3750 | 0.3620 | 0.0130 | 6.60 | MES | 25 | 15 | -5.78E-01 | -3.35E+00 |
| 4-CNNB[61] | Lepidocrocite | 0.239 | 49.00 | 0.3750 | 0.3530 | 0.0220 | 6.60 | MES | 25 | 15 | -2.78E-02 | -1.10E+00 |
| 4-CNNB[61] | Lepidocrocite | 0.600 | 49.00 | 0.3750 | 0.3190 | 0.0560 | 6.60 | MES | 25 | 15 | 3.75E-01 | -1.09E+00 |
| 4-CNNB[61] | Lepidocrocite | 1.198 | 49.00 | 0.3750 | 0.2700 | 0.1050 | 6.60 | MES | 25 | 15 | 5.05E-01 | -1.26E+00 |
| 4-CNNB[61] | Lepidocrocite | 1.798 | 49.00 | 0.3750 | 0.2230 | 0.1520 | 6.60 | MES | 25 | 15 | 5.56E-01 | -1.39E+00 |
| 4-CNNB[61] | Lepidocrocite | 2.388 | 49.00 | 0.3750 | 0.1820 | 0.1930 | 6.60 | MES | 25 | 15 | 5.28E-01 | -1.54E+00 |
| 4-CNNB[61] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3440 | 0.0310 | 7.97 | HEPES | 25 | 15 | 9.21E-01 | 8.07E-01 |
| 4-CNNB[61] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3660 | 0.0094 | 7.67 | HEPES | 25 | 15 | 3.05E-01 | 1.91E-01 |
| 4-CNNB[61] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3570 | 0.0180 | 7.50 | MOPS | 25 | 15 | -9.96E-02 | -2.14E-01 |
| 4-CNNB[61] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3640 | 0.0110 | 7.28 | MOPS | 25 | 15 | -8.18E-01 | -9.32E-01 |
| 4-CNNB[61] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3670 | 0.0084 | 7.00 | MOPS | 25 | 15 | -1.61E+00 | -1.73E+00 |
| 4-CNNB[61] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3750 | 0.0004 | 6.80 | MOPSO | 25 | 15 | -1.82E+00 | -1.93E+00 |
| 4-CNNB[61] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3730 | 0.0018 | 6.60 | MES | 25 | 15 | -2.26E+00 | -2.37E+00 |
| 4-CNNB[61] | Goethite | 0.025 | 51.00 | 0.3750 | 0.3670 | 0.0076 | 6.10 | MES | 25 | 15 | -3.56E+00 | -3.67E+00 |
| 4-CNNB[61] | Goethite | 0.020 | 51.00 | 0.3750 | 0.3680 | 0.0069 | 6.60 | MES | 25 | 15 | -2.26E+00 | -2.27E+00 |
| 4-CNNB[61] | Goethite | 0.110 | 51.00 | 0.3750 | 0.3660 | 0.0090 | 6.60 | MES | 25 | 15 | -3.19E-01 | -1.07E+00 |
| 4-CNNB[61] | Goethite | 0.220 | 51.00 | 0.3750 | 0.3540 | 0.0210 | 6.60 | MES | 25 | 15 | 5.00E-01 | -5.50E-01 |
| 4-CNNB[61] | Goethite | 0.551 | 51.00 | 0.3750 | 0.3220 | 0.0530 | 6.60 | MES | 25 | 15 | 1.03E+00 | -4.15E-01 |
| 4-CNNB[61] | Goethite | 1.100 | 51.00 | 0.3750 | 0.2740 | 0.1010 | 6.60 | MES | 25 | 15 | 1.46E+00 | -2.88E-01 |
| 4-CNNB[61] | Goethite | 1.651 | 51.00 | 0.3750 | 0.2330 | 0.1420 | 6.60 | MES | 25 | 15 | 1.66E+00 | -2.70E-01 |
| 4-CNNB[61] | Goethite | 2.196 | 51.00 | 0.3750 | 0.1910 | 0.1840 | 6.60 | MES | 25 | 15 | 1.83E+00 | -2.19E-01 |
| 4-CNNB[61] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | 1.99E-01 | -6.61E-01 | ||
| 4-AcNB[61] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -6.85E-02 | -9.28E-01 | ||
| 4-ClNB[61] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -5.47E-01 | -1.41E+00 | ||
| 4-BrNB[61] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -5.73E-01 | -1.43E+00 | ||
| NB[61] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -7.93E-01 | -1.65E+00 | ||
| 4-MeNB[61] | Goethite | 0.142 | 51.00 | 0.3750 | 6.60 | MES | 25 | 15 | -9.79E-01 | -1.84E+00 | ||
| 4-ClNB[62] | Goethite | 0.040 | 186.75 | 1.0000 | 0.8050 | 0.1950 | 7.00 | 1.05E+00 | -3.20E-01 | |||
| 4-ClNB[62] | Goethite | 7.516 | 16.10 | 1.0000 | 0.9260 | 0.0740 | 7.00 | 1.14E+00 | 0.00E+00 | |||
| 4-ClNB[62] | Ferrihydrite | 0.111 | 252.60 | 1.0000 | 0.6650 | 0.3350 | 7.00 | 1.05E+00 | -1.56E+00 | |||
| 4-ClNB[62] | Lepidocrocite | 2.384 | 60.40 | 1.0000 | 0.9250 | 0.0750 | 7.00 | 1.14E+00 | -8.60E-01 | |||
| 4-ClNB[63] | Goethite | 10.000 | 14.90 | 1.0000 | 7.20 | HEPES | 10 | 10 - 50 | 2.26E+00 | 8.00E-02 | ||
| 4-ClNB[63] | Goethite | 3.000 | 14.90 | 1.0000 | 7.20 | HEPES | 10 | 10 - 50 | 2.38E+00 | 7.30E-01 | ||
| 4-ClNB[63] | Lepidocrocite | 2.700 | 16.20 | 1.0000 | 7.20 | HEPES | 10 | 10 - 50 | 9.20E-01 | -7.20E-01 | ||
| 4-ClNB[63] | Lepidocrocite | 10.000 | 16.20 | 1.0000 | 7.20 | HEPES | 10 | 10 - 50 | 1.03E+00 | -1.18E+00 | ||
| 4-ClNB[64] | Goethite | 0.325 | 140.00 | 1.0000 | 7.00 | Bicarbonate | 10 | 100 | 1.14E+00 | -1.79E+00 | ||
| 4-ClNB[64] | Goethite | 0.325 | 140.00 | 1.0000 | 6.50 | Bicarbonate | 10 | 100 | 1.11E+00 | -2.10E+00 | ||
| NB[65] | Goethite | 0.500 | 30.70 | 0.1000 | 0.1120 | 0.0090 | 6.00 | MES | 25 | 12 | -1.42E+00 | -2.61E+00 |
| NB[65] | Goethite | 0.500 | 30.70 | 0.5000 | 0.5150 | 0.0240 | 6.00 | MES | 25 | 15 | -7.45E-01 | -1.93E+00 |
| NB[65] | Goethite | 0.500 | 30.70 | 1.0000 | 1.0280 | 0.0140 | 6.00 | MES | 25 | 19 | -7.45E-01 | -1.93E+00 |
| NB[65] | Goethite | 1.000 | 30.70 | 0.1000 | 0.0960 | 0.0260 | 6.00 | MES | 25 | 13 | -1.12E+00 | -2.61E+00 |
| NB[65] | Goethite | 1.000 | 30.70 | 0.5000 | 0.4890 | 0.0230 | 6.00 | MES | 25 | 14 | -5.53E-01 | -2.04E+00 |
| NB[65] | Goethite | 1.000 | 30.70 | 1.0000 | 0.9870 | 0.0380 | 6.00 | MES | 25 | 19 | -2.52E-01 | -1.74E+00 |
| NB[65] | Goethite | 2.000 | 30.70 | 0.1000 | 0.0800 | 0.0490 | 6.00 | MES | 25 | 11 | -8.86E-01 | -2.67E+00 |
| NB[65] | Goethite | 2.000 | 30.70 | 0.6000 | 0.4890 | 0.0640 | 6.00 | MES | 25 | 14 | -1.08E-01 | -1.90E+00 |
| NB[65] | Goethite | 2.000 | 30.70 | 1.1000 | 0.9870 | 0.0670 | 6.00 | MES | 25 | 14 | 2.30E-01 | -1.56E+00 |
| NB[65] | Goethite | 4.000 | 30.70 | 0.1000 | 0.0600 | 0.0650 | 6.00 | MES | 25 | 11 | -8.89E-01 | -2.98E+00 |
| NB[65] | Goethite | 4.000 | 30.70 | 0.6000 | 0.3960 | 0.1550 | 6.00 | MES | 25 | 17 | 1.43E-01 | -1.95E+00 |
| NB[65] | Goethite | 4.000 | 30.70 | 1.0000 | 0.8360 | 0.1450 | 6.00 | MES | 25 | 16 | 4.80E-01 | -1.61E+00 |
| NB[65] | Goethite | 4.000 | 30.70 | 5.6000 | 5.2110 | 0.3790 | 6.00 | MES | 25 | 15 | 1.17E+00 | -9.19E-01 |
| NB[65] | Goethite | 1.000 | 30.70 | 0.1000 | 0.0870 | 0.0300 | 6.50 | MES | 25 | 5.5 | -1.74E-01 | -1.66E+00 |
| NB[65] | Goethite | 1.000 | 30.70 | 0.5000 | 0.4920 | 0.0300 | 6.50 | MES | 25 | 15 | 3.64E-01 | -1.12E+00 |
| NB[65] | Goethite | 1.000 | 30.70 | 1.0000 | 0.9390 | 0.0650 | 6.50 | MES | 25 | 18 | 6.70E-01 | -8.17E-01 |
| NB[65] | Goethite | 2.000 | 30.70 | 0.1000 | 0.0490 | 0.0730 | 6.50 | MES | 25 | 5.2 | 3.01E-01 | -1.49E+00 |
| NB[65] | Goethite | 2.000 | 30.70 | 0.5000 | 0.4640 | 0.0710 | 6.50 | MES | 25 | 14 | 8.85E-01 | -9.03E-01 |
| NB[65] | Goethite | 2.000 | 30.70 | 1.0000 | 0.9130 | 0.1280 | 6.50 | MES | 25 | 16 | 1.12E+00 | -6.64E-01 |
| NB[65] | Goethite | 1.000 | 30.70 | 0.1000 | 0.0630 | 0.0480 | 7.00 | MOPS | 25 | 5.3 | 6.12E-01 | -8.75E-01 |
| NB[65] | Goethite | 1.000 | 30.70 | 0.5000 | 0.4690 | 0.0520 | 7.00 | MOPS | 25 | 9 | 1.51E+00 | 2.07E-02 |
| NB[65] | Goethite | 1.000 | 30.70 | 1.0000 | 0.9360 | 0.1090 | 7.00 | MOPS | 25 | 18 | 1.33E+00 | -1.53E-01 |
| NB[65] | Goethite | 2.000 | 30.70 | 0.1000 | 0.0290 | 0.0880 | 7.00 | MOPS | 25 | 12 | 6.85E-01 | -1.10E+00 |
| NB[65] | Goethite | 2.000 | 30.70 | 0.5000 | 0.3950 | 0.1450 | 7.00 | MOPS | 25 | 15 | 1.59E+00 | -1.95E-01 |
| Notes: a The NACs are Nitrobenzene (NB), 4-chloronitrobenzene(4-ClNB), 4-cyanonitrobenzene (4-CNNB), 4-acetylnitrobenzene (4-AcNB), 4-bromonitrobenzene (4-BrNB), 4-nitrotoluene (4-MeNB). b Initial aqueous Fe(II). c Aqueous Fe(II) after 24h of equilibration. d Difference between b and c. e Initial nominal NAC concentration. f Pseudo-first order rate constant. g Surface area normalized rate constant calculated as kObs / (surface area × mineral loading). | ||||||||||||
Iron(II) can be complexed by a myriad of organic ligands and may thereby become more reactive towards MCs and other pollutants. The reactivity of an Fe(II)-organic complex depends on the relative preference of the organic ligand for Fe(III) versus Fe(II)[44]. Since the majority of naturally occurring ligands complex Fe(III) more strongly than Fe(II), the reduction potential of the resulting Fe(III) complex is lower than that of aqueous Fe(III); therefore, complexation by organic ligands often renders Fe(II) a stronger reductant thermodynamically[66]. The reactivity of dissolved Fe(II)-organic complexes towards NACs/MCs has been investigated. The intrinsic, second-order rate constants and one electron reduction potentials are listed in Table 2.
In addition to forming organic complexes, iron is ubiquitous in minerals. Iron-bearing minerals play an important role in controlling the environmental fate of contaminants through adsorption[67][68] and reduction[69] processes. Studies have shown that aqueous Fe(II) itself cannot reduce NACs/MCs at circumneutral pH[59][49] but in the presence of an iron oxide (e.g., goethite, hematite, lepidocrocite, ferrihydrite, or magnetite), NACs[61][59][64][60][70] and MCs such as TNT[39], RDX[49], DNAN[54], and NG[50] can be rapidly reduced. Unlike ferric oxides, Fe(II)-bearing minerals including clays[70][71][72][73][74][75][76], green rust[52][53], mackinawite[60][54][58] and pyrite[60][51] do not need aqueous Fe(II) to be reactive toward NACs/MCs. However, upon oxidation, sulfate green rust was converted into lepidocrocite[53], and mackinawite into goethite[58], suggesting that aqueous Fe(II) coupled to Fe(III) oxides might be at least partially responsible for continued degradation of NACs/MCs in the subsurface once the parent reductant (e.g., green rust or iron sulfide) oxidizes.
The reaction conditions and rate constants for a list of studies on MC reduction by iron oxide-aqueous Fe(II) redox couples and by other Fe(II)-containing minerals are shown in Table 3[39][52][49][54][51][55][56]. Unlike hydroquinones and Fe(II) complexes, where second-order rate constants can be readily calculated, the reduction rate constants of NACs/MCs in mineral suspensions are often specific to the experimental conditions used and are usually reported as BET surface area-normalized reduction rate constants (kSA). In the case of iron oxide-Fe(II) redox couples, reduction rate constants have been shown to increase with pH (specifically, with [OH– ]2) and aqueous Fe(II) concentration, both of which correspond to a decrease in the system's reduction potential[61][77][56].
For minerals that contain structural iron(II) and can reduce pollutants in the absence of aqueous Fe(II), the observed rates of reduction increased with increasing structural Fe(II) content, as seen with iron-bearing clays[72][73] and green rust[52]. This dependency on Fe(II) content allows for the derivation of second-order rate constants, as shown on Table 3 for the reduction of RDX by green rust[52], and the development of reduction potential (EH)-based models[72][78][79][80], where EH represents the reduction potential of the iron-bearing clays. Iron-bearing expandable clay minerals represent a special case, which in addition to reduction can remove NACs/MCs through adsorption. This is particularly important for planar NACs/MCs that contain multiple electron-withdrawing nitro groups and can form strong electron donor-acceptor (EDA) complexes with the clay surface[70][74][75].
Although the second-order rate constants derived for Fe(II)-bearing minerals may allow comparison among different studies, they may not reflect changes in reactivity due to variations in surface area, pH, and the presence of ions. Anions such as bicarbonate[52][55][81] and phosphate[52][82] are known to decrease the reactivity of iron oxides-Fe(II) redox couples and green rust. Sulfite has also been shown to decrease the reactivity of hematite-Fe(II) towards the deprotonated form of NTO (Table 3)[56]. Exchanging cations in iron-bearing clays can change the reactivity of these minerals by up to 7-fold[70]. Thus, more comprehensive models are needed to account for the complexities in the subsurface environment.
The reduction of NACs has been widely studied in the presence of different iron minerals, pH, and Fe(II)(aq) concentrations (Table 4)[61][59][64][60][70]. Only selected NACs are included in Table 4. For more information on other NACs and ferruginous reductants, please refer to the cited references.
References
- ^ Rosenberry, D. O., Duque, C., and Lee, D. R., 2020. History and Evolution of Seepage Meters for Quantifying Flow between Groundwater and Surface Water: Part 1 – Freshwater Settings. Earth-Science Reviews, 204(103167). doi: 10.1016/j.earscirev.2020.103167.
- ^ Duque, C., Russoniello, C. J., and Rosenberry, D. O., 2020. History and Evolution of Seepage Meters for Quantifying Flow between Groundwater and Surface Water: Part 2 – Marine Settings and Submarine Groundwater Discharge. Earth-Science Reviews, 204 ( 103168). doi: 10.1016/j.earscirev.2020.103168.
- ^ Constantz, J., 2008. Heat as a Tracer to Determine Streambed Water Exchanges. Water Resources Research, 44 (4).doi: 10.1029/2008WR006996.Open Access Article
- ^ Briggs, M. A., Jackson, K. E., Liu, F., Moore, E. M., Bisson, A., Helton, A. M., 2022. Exploring Local Riverbank Sediment Controls on the Occurrence of Preferential Groundwater Discharge Points. Water, 14(1). doi: 10.3390/w14010011 Open Access Article.
- ^ Briggs, M. A., Wang, C., Day-Lewis, F. D., Williams, K. H., Dong, W., Lane, J. W., 2019. Return Flows from Beaver Ponds Enhance Floodplain-to-River Metals Exchange in Alluvial Mountain Catchments. Science of the Total Environment, 685, pp. 357–369. doi: 10.1016/j.scitotenv.2019.05.371. Open Access Manuscript
- ^ Mangel, A. R., Dawson, C. B., Rey, D. M., Briggs, M. A., 2022. Drone Applications in Hydrogeophysics: Recent Examples and a Vision for the Future. The Leading Edge, 41 (8), pp. 540–547. doi: 10.1190/tle41080540.
- ^ 7.0 7.1 Selker, J. S., Thévenaz, L., Huwald, H., Mallet, A., Luxemburg, W., van de Giesen, N., Stejskal, M., Zeman, J., Westhoff, M., Parlange, M. B., 2006. Distributed Fiber-Optic Temperature Sensing for Hydrologic Systems. Water Resources Research, 42 (12). doi: 10.1029/2006WR005326. Open Access Article
- ^ Tyler, S. W., Selker, J. S., Hausner, M. B., Hatch, C. E., Torgersen, T., Thodal, C. E., Schladow, S. G., 2009. Environmental Temperature Sensing Using Raman Spectra DTS Fiber-Optic Methods. Water Resources Research, 45(4). doi: 10.1029/2008WR007052. Open Access Article
- ^ Schumacher, B.A., 2002. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments. U.S. EPA, Ecological Risk Assessment Support Center. Free download.
- ^ Schmidt, M.W.I., and Noack, A.G., 2000. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Global Biogeochemical Cycles, 14(3), pp. 777–793. DOI: 10.1029/1999GB001208 Open access article.
- ^ 11.0 11.1 Klüpfel, L., Keiluweit, M., Kleber, M., and Sander, M., 2014. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environmental Science and Technology, 48(10), pp. 5601–5611. DOI: 10.1021/es500906d
- ^ Xin, D., Xian, M., and Chiu, P.C., 2019. New methods for assessing electron storage capacity and redox reversibility of biochar. Chemosphere, 215, 827–834. DOI: 10.1016/j.chemosphere.2018.10.080
- ^ Saquing, J.M., Yu, Y.-H., and Chiu, P.C., 2016. Wood-Derived Black Carbon (Biochar) as a Microbial Electron Donor and Acceptor. Environmental Science and Technology Letters, 3(2), pp. 62–66. DOI: 10.1021/acs.estlett.5b00354
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedXin2022 - ^ Sparks, D.L., 2003. Environmental Soil Chemistry, 2nd Edition. Elsevier Science and Technology Books. DOI: 10.1016/B978-0-12-656446-4.X5000-2
- ^ 16.00 16.01 16.02 16.03 16.04 16.05 16.06 16.07 16.08 16.09 16.10 16.11 16.12 16.13 16.14 16.15 16.16 16.17 16.18 16.19 16.20 16.21 16.22 16.23 16.24 16.25 16.26 16.27 16.28 16.29 16.30 16.31 16.32 16.33 16.34 16.35 16.36 16.37 16.38 16.39 16.40 16.41 16.42 16.43 16.44 16.45 16.46 16.47 16.48 16.49 16.50 16.51 16.52 16.53 16.54 16.55 16.56 Cite error: Invalid
<ref>tag; no text was provided for refs namedSchwarzenbach1990 - ^ 17.0 17.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedDunnivant1992 - ^ Scott, D.T., McKnight, D.M., Blunt-Harris, E.L., Kolesar, S.E., and Lovley, D.R., 1998. Quinone Moieties Act as Electron Acceptors in the Reduction of Humic Substances by Humics-Reducing Microorganisms. Environmental Science and Technology, 32(19), pp. 2984–2989. DOI: 10.1021/es980272q
- ^ Cory, R.M., and McKnight, D.M., 2005. Fluorescence Spectroscopy Reveals Ubiquitous Presence of Oxidized and Reduced Quinones in Dissolved Organic Matter. Environmental Science & Technology, 39(21), pp 8142–8149. DOI: 10.1021/es0506962
- ^ Fimmen, R.L., Cory, R.M., Chin, Y.P., Trouts, T.D., and McKnight, D.M., 2007. Probing the oxidation–reduction properties of terrestrially and microbially derived dissolved organic matter. Geochimica et Cosmochimica Acta, 71(12), pp. 3003–3015. DOI: 10.1016/j.gca.2007.04.009
- ^ Struyk, Z., and Sposito, G., 2001. Redox properties of standard humic acids. Geoderma, 102(3-4), pp. 329–346. DOI: 10.1016/S0016-7061(01)00040-4
- ^ Ratasuk, N., and Nanny, M.A., 2007. Characterization and Quantification of Reversible Redox Sites in Humic Substances. Environmental Science and Technology, 41(22), pp. 7844–7850. DOI: 10.1021/es071389u
- ^ Aeschbacher, M., Sander, M., and Schwarzenbach, R.P., 2010. Novel Electrochemical Approach to Assess the Redox Properties of Humic Substances. Environmental Science and Technology, 44(1), pp. 87–93. DOI: 10.1021/es902627p
- ^ Aeschbacher, M., Vergari, D., Schwarzenbach, R.P., and Sander, M., 2011. Electrochemical Analysis of Proton and Electron Transfer Equilibria of the Reducible Moieties in Humic Acids. Environmental Science and Technology, 45(19), pp. 8385–8394. DOI: 10.1021/es201981g
- ^ Bauer, I., and Kappler, A., 2009. Rates and Extent of Reduction of Fe(III) Compounds and O2 by Humic Substances. Environmental Science and Technology, 43(13), pp. 4902–4908. DOI: 10.1021/es900179s
- ^ Maurer, F., Christl, I. and Kretzschmar, R., 2010. Reduction and Reoxidation of Humic Acid: Influence on Spectroscopic Properties and Proton Binding. Environmental Science and Technology, 44(15), pp. 5787–5792. DOI: 10.1021/es100594t
- ^ Walpen, N., Schroth, M.H., and Sander, M., 2016. Quantification of Phenolic Antioxidant Moieties in Dissolved Organic Matter by Flow-Injection Analysis with Electrochemical Detection. Environmental Science and Technology, 50(12), pp. 6423–6432. DOI: 10.1021/acs.est.6b01120 Open access article.
- ^ Aeschbacher, M., Graf, C., Schwarzenbach, R.P., and Sander, M., 2012. Antioxidant Properties of Humic Substances. Environmental Science and Technology, 46(9), pp. 4916–4925. DOI: 10.1021/es300039h
- ^ Nurmi, J.T., and Tratnyek, P.G., 2002. Electrochemical Properties of Natural Organic Matter (NOM), Fractions of NOM, and Model Biogeochemical Electron Shuttles. Environmental Science and Technology, 36(4), pp. 617–624. DOI: 10.1021/es0110731
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedOh2013 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedOh2009 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedXu2015 - ^ Xin, D., 2021. Understanding the Electron Storage Capacity of Pyrogenic Black Carbon: Origin, Redox Reversibility, Spatial Distribution, and Environmental Applications. Doctoral Thesis, University of Delaware. Free download.
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedLuan2010 - ^ 35.0 35.1 35.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedMurillo-Gelvez2021 - ^ 36.00 36.01 36.02 36.03 36.04 36.05 36.06 36.07 36.08 36.09 36.10 36.11 36.12 36.13 36.14 36.15 36.16 Cite error: Invalid
<ref>tag; no text was provided for refs namedMurillo-Gelvez2019 - ^ 37.0 37.1 37.2 37.3 37.4 37.5 Cite error: Invalid
<ref>tag; no text was provided for refs namedHartenbach2008 - ^ 38.0 38.1 38.2 38.3 38.4 38.5 38.6 Cite error: Invalid
<ref>tag; no text was provided for refs namedSchwarzenbach2016 - ^ 39.00 39.01 39.02 39.03 39.04 39.05 39.06 39.07 39.08 39.09 39.10 39.11 39.12 39.13 39.14 39.15 39.16 39.17 39.18 39.19 Cite error: Invalid
<ref>tag; no text was provided for refs namedHofstetter1999 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedKwon2008 - ^ Riefler, R.G., and Smets, B.F., 2000. Enzymatic Reduction of 2,4,6-Trinitrotoluene and Related Nitroarenes: Kinetics Linked to One-Electron Redox Potentials. Environmental Science and Technology, 34(18), pp. 3900–3906. DOI: 10.1021/es991422f
- ^ 42.00 42.01 42.02 42.03 42.04 42.05 42.06 42.07 42.08 42.09 42.10 42.11 Salter-Blanc, A.J., Bylaska, E.J., Johnston, H.J., and Tratnyek, P.G., 2015. Predicting Reduction Rates of Energetic Nitroaromatic Compounds Using Calculated One-Electron Reduction Potentials. Environmental Science and Technology, 49(6), pp. 3778–3786. DOI: 10.1021/es505092s Open access article.
- ^ 43.0 43.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedNaka2008 - ^ 44.0 44.1 44.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedKim2009 - ^ 45.00 45.01 45.02 45.03 45.04 45.05 45.06 45.07 45.08 45.09 45.10 Gao, Y., Zhong, S., Torralba-Sanchez, T.L., Tratnyek, P.G., Weber, E.J., Chen, Y., and Zhang, H., 2021. Quantitative structure activity relationships (QSARs) and machine learning models for abiotic reduction of organic compounds by an aqueous Fe(II) complex. Water Research, 192, p. 116843. DOI: 10.1016/j.watres.2021.116843
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedNaka2006 - ^ 47.0 47.1 47.2 47.3 Uchimiya, M., Gorb, L., Isayev, O., Qasim, M.M., and Leszczynski, J., 2010. One-electron standard reduction potentials of nitroaromatic and cyclic nitramine explosives. Environmental Pollution, 158(10), pp. 3048–3053. DOI: 10.1016/j.envpol.2010.06.033
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedKim2007 - ^ 49.00 49.01 49.02 49.03 49.04 49.05 49.06 49.07 49.08 49.09 49.10 49.11 49.12 49.13 49.14 49.15 Gregory, K.B., Larese-Casanova, P., Parkin, G.F., and Scherer, M.M., 2004. Abiotic Transformation of Hexahydro-1,3,5-trinitro-1,3,5-triazine by FeII Bound to Magnetite. Environmental Science and Technology, 38(5), pp. 1408–1414. DOI: 10.1021/es034588w
- ^ 50.0 50.1 Oh, S.-Y., Cha, D.K., Kim, B.J., and Chiu, P.C., 2004. Reduction of Nitroglycerin with Elemental Iron: Pathway, Kinetics, and Mechanisms. Environmental Science and Technology, 38(13), pp. 3723–3730. DOI: 10.1021/es0354667
- ^ 51.0 51.1 51.2 51.3 51.4 Oh, S.-Y., Chiu, P.C., and Cha, D.K., 2008. Reductive transformation of 2,4,6-trinitrotoluene, hexahydro-1,3,5-trinitro-1,3,5-triazine, and nitroglycerin by pyrite and magnetite. Journal of hazardous materials, 158(2-3), pp. 652–655. DOI: 10.1016/j.jhazmat.2008.01.078
- ^ 52.0 52.1 52.2 52.3 52.4 52.5 52.6 52.7 Cite error: Invalid
<ref>tag; no text was provided for refs namedLarese-Casanova2008 - ^ 53.0 53.1 53.2 53.3 Khatiwada, R., Root, R.A., Abrell, L., Sierra-Alvarez, R., Field, J.A., and Chorover, J., 2018. Abiotic reduction of insensitive munition compounds by sulfate green rust. Environmental Chemistry, 15(5), pp. 259–266. DOI: 10.1071/EN17221
- ^ 54.0 54.1 54.2 54.3 54.4 54.5 Berens, M.J., Ulrich, B.A., Strehlau, J.H., Hofstetter, T.B., and Arnold, W.A., 2019. Mineral identity, natural organic matter, and repeated contaminant exposures do not affect the carbon and nitrogen isotope fractionation of 2,4-dinitroanisole during abiotic reduction. Environmental Science: Processes and Impacts, 21(1), pp. 51-62. DOI: 10.1039/C8EM00381E
- ^ 55.0 55.1 55.2 55.3 55.4 Strehlau, J.H., Berens, M.J., and Arnold, W.A., 2018. Mineralogy and buffer identity effects on RDX kinetics and intermediates during reaction with natural and synthetic magnetite. Chemosphere, 213, pp. 602–609. DOI: 10.1016/j.chemosphere.2018.09.139
- ^ 56.00 56.01 56.02 56.03 56.04 56.05 56.06 56.07 56.08 56.09 56.10 Cárdenas-Hernandez, P.A., Anderson, K.A., Murillo-Gelvez, J., di Toro, D.M., Allen, H.E., Carbonaro, R.F., and Chiu, P.C., 2020. Reduction of 3-Nitro-1,2,4-Triazol-5-One (NTO) by the Hematite–Aqueous Fe(II) Redox Couple. Environmental Science and Technology, 54(19), pp. 12191–12201. DOI: 10.1021/acs.est.0c03872
- ^ 57.00 57.01 57.02 57.03 57.04 57.05 57.06 57.07 57.08 57.09 57.10 57.11 57.12 57.13 57.14 Cite error: Invalid
<ref>tag; no text was provided for refs namedTong2021 - ^ 58.0 58.1 58.2 58.3 58.4 58.5 58.6 58.7 58.8 58.9 Menezes, O., Yu, Y., Root, R.A., Gavazza, S., Chorover, J., Sierra-Alvarez, R., and Field, J.A., 2021. Iron(II) monosulfide (FeS) minerals reductively transform the insensitive munitions compounds 2,4-dinitroanisole (DNAN) and 3-nitro-1,2,4-triazol-5-one (NTO). Chemosphere, 285, p. 131409. DOI: 10.1016/j.chemosphere.2021.131409
- ^ 59.0 59.1 59.2 59.3 59.4 Cite error: Invalid
<ref>tag; no text was provided for refs namedKlausen1995 - ^ 60.0 60.1 60.2 60.3 60.4 60.5 60.6 Cite error: Invalid
<ref>tag; no text was provided for refs namedElsner2004 - ^ 61.00 61.01 61.02 61.03 61.04 61.05 61.06 61.07 61.08 61.09 61.10 61.11 61.12 61.13 61.14 61.15 61.16 61.17 61.18 61.19 61.20 61.21 61.22 61.23 61.24 61.25 61.26 61.27 61.28 61.29 61.30 61.31 61.32 61.33 61.34 61.35 61.36 61.37 61.38 61.39 61.40 61.41 61.42 61.43 61.44 61.45 61.46 61.47 61.48 61.49 61.50 61.51 61.52 61.53 61.54 61.55 61.56 61.57 61.58 61.59 61.60 61.61 61.62 61.63 61.64 61.65 61.66 61.67 Cite error: Invalid
<ref>tag; no text was provided for refs namedColón2006 - ^ 62.0 62.1 62.2 62.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedJones2016 - ^ 63.0 63.1 63.2 63.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedFan2016 - ^ 64.0 64.1 64.2 64.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedStrehlau2016 - ^ 65.00 65.01 65.02 65.03 65.04 65.05 65.06 65.07 65.08 65.09 65.10 65.11 65.12 65.13 65.14 65.15 65.16 65.17 65.18 65.19 65.20 65.21 65.22 65.23 Cite error: Invalid
<ref>tag; no text was provided for refs namedStewart2018 - ^ Strathmann, T.J., 2011. Redox Reactivity of Organically Complexed Iron(II) Species with Aquatic Contaminants. Aquatic Redox Chemistry, American Chemical Society,1071(14), pp. 283-313. DOI: 10.1021/bk-2011-1071.ch014
- ^ Linker, B.R., Khatiwada, R., Perdrial, N., Abrell, L., Sierra-Alvarez, R., Field, J.A., and Chorover, J., 2015. Adsorption of novel insensitive munitions compounds at clay mineral and metal oxide surfaces. Environmental Chemistry, 12(1), pp. 74–84. DOI: 10.1071/EN14065
- ^ Jenness, G.R., Giles, S.A., and Shukla, M.K., 2020. Thermodynamic Adsorption States of TNT and DNAN on Corundum and Hematite. The Journal of Physical Chemistry C, 124(25), pp. 13837–13844. DOI: 10.1021/acs.jpcc.0c04512
- ^ Gorski, C.A., and Scherer, M.M., 2011. Fe2+ Sorption at the Fe Oxide-Water Interface: A Revised Conceptual Framework. Aquatic Redox Chemistry, American Chemical Society, 1071(15), pp. 315–343. DOI: 10.1021/bk-2011-1071.ch015
- ^ 70.0 70.1 70.2 70.3 70.4 Cite error: Invalid
<ref>tag; no text was provided for refs namedHofstetter2006 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedSchultz2000 - ^ 72.0 72.1 72.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedLuan2015a - ^ 73.0 73.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedLuan2015b - ^ 74.0 74.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedHofstetter2003 - ^ 75.0 75.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedNeumann2008 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedHofstetter2008 - ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedGorski2016 - ^ Gorski, C.A., Aeschbacher, M., Soltermann, D., Voegelin, A., Baeyens, B., Marques Fernandes, M., Hofstetter, T.B., and Sander, M., 2012. Redox Properties of Structural Fe in Clay Minerals. 1. Electrochemical Quantification of Electron-Donating and -Accepting Capacities of Smectites. Environmental Science and Technology, 46(17), pp. 9360–9368. DOI: 10.1021/es3020138
- ^ Gorski, C.A., Klüpfel, L., Voegelin, A., Sander, M., and Hofstetter, T.B., 2012. Redox Properties of Structural Fe in Clay Minerals. 2. Electrochemical and Spectroscopic Characterization of Electron Transfer Irreversibility in Ferruginous Smectite, SWa-1. Environmental Science and Technology, 46(17), pp. 9369–9377. DOI: 10.1021/es302014u
- ^ Gorski, C.A., Klüpfel, L.E., Voegelin, A., Sander, M. and Hofstetter, T.B., 2013. Redox Properties of Structural Fe in Clay Minerals: 3. Relationships between Smectite Redox and Structural Properties. Environmental Science and Technology, 47(23), pp. 13477–13485. DOI: 10.1021/es403824x
- ^ Chen, G., Hofstetter, T.B., and Gorski, C.A., 2020. Role of Carbonate in Thermodynamic Relationships Describing Pollutant Reduction Kinetics by Iron Oxide-Bound Fe2+. Environmental Science and Technology, 54(16), pp. 10109–10117. DOI: 10.1021/acs.est.0c02959
- ^ Bocher, F., Géhin, A., Ruby, C., Ghanbaja, J., Abdelmoula, M., and Génin, J.M.R., 2004. Coprecipitation of Fe(II–III) hydroxycarbonate green rust stabilised by phosphate adsorption. Solid State Sciences, 6(1), pp. 117–124. DOI: 10.1016/j.solidstatesciences.2003.10.004