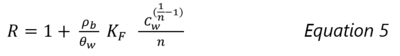Difference between revisions of "Sorption of Organic Contaminants"
(Tag: Visual edit) |
(Tag: Visual edit) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 6: | Line 6: | ||
*[[Chlorinated Solvents]] | *[[Chlorinated Solvents]] | ||
| − | *[[Petroleum Hydrocarbons]] | + | *[[Petroleum Hydrocarbons (PHCs)]] |
| + | |||
| + | |||
| + | '''Contributor(s):''' [[Richelle Allen-King|Dr. Richelle Allen-King]] | ||
| − | |||
'''Key Resource(s)''': | '''Key Resource(s)''': | ||
Revision as of 21:45, 26 April 2022
Nonionic and nonpolar organic contaminants including chlorinated solvents and petroleum hydrocarbons can become associated with solid phase organic matter in the soil, sediment, and rock, slowing contaminant migration in groundwater and reducing the efficacy of many remedial technologies. When amorphous organic matter is the primary sorbent, sorption is linear and distribution coefficients can be estimated from empirical correlations with contaminant properties (aqueous solubility or octanol-water partition coefficient) and site specific measurements of solid phase organic carbon. However, when thermally altered carbonaceous materials (TACMs) are present, sorption may be non-linear and the empirical correlations may substantially underestimate sorption at low contaminant concentrations.
Related Article(s):
Contributor(s): Dr. Richelle Allen-King
Key Resource(s):
Introduction
Sorption is a non-mechanistic term that describes uptake of a contaminant by a solid phase sorbent (soil, sediment, rock or an engineered material such as activated carbon). This article focuses on sorption of nonionic and nonpolar organic contaminants and their associations with subsurface materials. For these contaminants, sorption is almost exclusively associated with organic matter in the solids and is a reversible process. Sorption of ionic and polar organic contaminants is not described in this article.

In this article, the term ‘sorption’ includes all processes that cause the dissolved organic compound (sorbate) to become associated with the solid phase (sorbent). For nonpolar organic compounds, sorption occurs by either adsorption or absorption. Absorption is a volume-based process whereby the sorbate diffuses into the organic matter, analogous to the way that organic contaminants partition between immiscible oil and aqueous phases. In contrast, adsorption describes the interaction between the contaminant and the sorbent surfaces.
Measuring Sorption
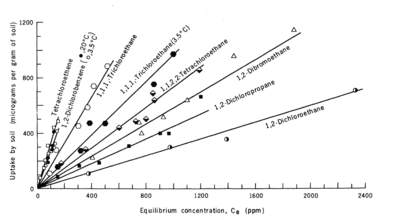
Sorption can be measured in the laboratory by placing a known mass of the contaminant, water, and uncontaminated sediment in a batch reactor (Figure 1a). The system is mixed for a specific duration (contact time) and then the aqueous and sorbed contaminant concentrations are determined. This process is repeated, adding a range of different initial contaminant masses to achieve a range of aqueous contaminant concentrations. When the experiment is run at constant temperature until the concentrations in the solid and liquid phases approach equilibrium, the resulting data plot is termed an isotherm. Figure 1b shows linear sorption isotherms for eight compounds measured in one soil.
The time required to reach equilibrium can vary depending on the contaminant and sorbent. In studies with aquifer material from Canadian Forces Base Borden, tetrachloroethene (PCE) sorption reached equilibrium within 2 - 4 weeks, but tetrachlorobenzene did not reach equilibrium after 100 days. Powdered (pulverized) samples equilibrated more quickly indicating mass transfer within the grains was limiting[3].
In some cases, the experimental data show an approximately linear relationship between aqueous and sorbed concentration, and the experimental results can be summarized in the form of a partition or distribution coefficient, Kd:
where CS is the solid (sorbed) concentration and Cw is the aqueous (dissolved) concentration. By convention, Cs has units of µg/g and Cs has units of µg/mL, so Kd has units of mL/g.
When the relationship between Cs and Cw is non-linear, the experimental data are commonly represented by the Freundlich isotherm equation:
where KF and n are empirically estimated coefficients specific to the sorbate, sorbent and equation used.
In most cases, absorption processes follow a linear isotherm. However, adsorption processes often follow a nonlinear relationship between the sorbed and dissolved contaminant concentrations. When both absorption and adsorption occur, a non-linear relationship is used to represent the sorption results.
Impact on Groundwater Transport
Sorption reduces the amount of contaminant in the aqueous phase, slowing contaminant migration. Sorbed contaminants are also less available for biological or chemical degradation reactions, which will reduce the effectiveness of many remedial technologies.
The average rate that a contaminant migrates in groundwater is reduced because the sorbed portion of the contaminant does not migrate. The impact of sorption on the average contaminant transport velocity can be estimated using a Retardation Factor (R):
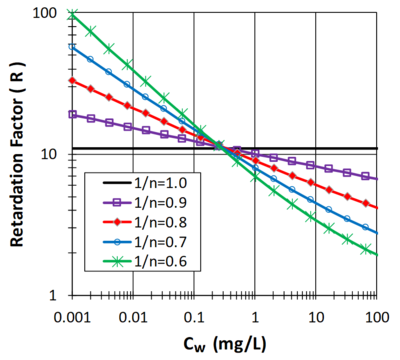
When sorption of the contaminant is described by a linear isotherm and sorption processes are fast relative to water flow, R can be estimated as:
where ρb is the bulk density of the solid phase and θw is the water filled porosity. When sorption is non-linear and is described with the Freundlich isotherm equation, R can be estimated as:
Figure 2 illustrates the effect of Cw on R when 1/n is less than 1 and ρb = 1.5 g/cm3, θw = 0.3 mL/cm3and KF = 2. When 1/n is less than 1, sorption has limited effect in slowing the migration of a high concentration plume (Cw > 10 mg/L). However, as the aquifer is remediated and contaminant concentrations drop below 0.1 mg/L, non-linear sorption has a much more pronounced impact, slowing aquifer cleanup.
Kd Estimation Methods
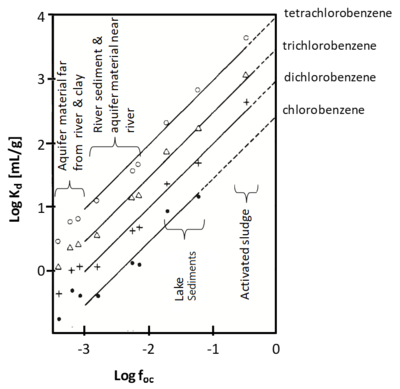
Multiple studies[4][5][6] showed that sorption of nonpolar organic contaminants is typically proportional to the organic carbon content of the solid phase (Figure 3). When this occurs, an organic carbon normalized partitioning coefficient, Koc, is used:
where foc is the fractional organic carbon content of the soil, sediment or rock. This approach empirically separates variations in Kd according to variations in the organic matter concentration, an important property of the sorbent, and the Koc, which is intended to capture differences in the chemical properties of the contaminant.
Experimental investigations (summarized in Allen-King et al., 2002[7]) with a variety of sorbents and contaminants has shown that Koc values are correlated with contaminant hydrophobicity. This allows Koc to be estimated using empirical correlations with the contaminant aqueous solubility or octanol-water partition coefficient (Kow) (Figure 4). Using this approach, Kd can be estimated using site specific measurements of foc and literature values of contaminant properties.
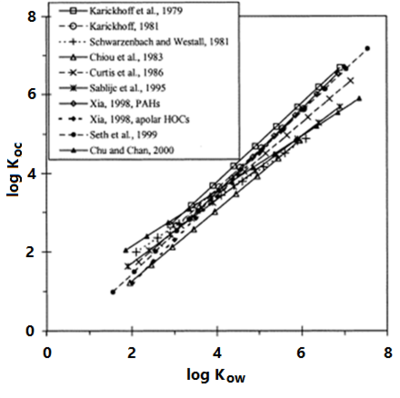
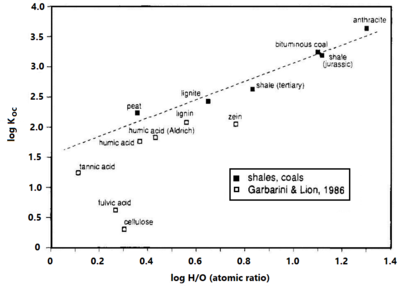
Effect of Carbon Type
Research reviewed by Allen-King et al. (2002)[7] Cornelissen et al. (2004)[9], and Huang et al. (2003)[10] indicates that some types of organic matter are much stronger sorbents than others. Experimental results show that thermally altered carbonaceous materials (TACMs), such as coals, kerogen and black carbons, will sorb greater concentrations of nonpolar organic contaminants than unaltered organic materials per gram of carbon (Figure 5), especially at low concentrations relative to solubility. TACMs are more condensed than their unaltered precursors, and have a lower oxygen content, and higher aromatic content than unaltered materials. Sorbed concentrations to TACM can be 10 to 100 times greater at equilibrium than the amount sorbed to less condensed forms of organic matter on a per-gram-carbon basis.
Sorption to TACMs is dominated by adsorptive behavior with strong sorption at low aqueous concentrations, with progressively weaker sorption at higher concentrations[9][7][10]. This effect is commonly represented with a Freundlich isotherm with the Freundlich exponent <1.
TACMs are typically associated with shale rocks or coal containing kerogen that has been buried and lithified, or with soils containing char or soot from incomplete combustion. This material may be present in consolidated rock or in sediments that contain these materials[11][12]. However, weathering of these materials appears to diminish adsorption[8].
How to Estimate Sorption
For high contaminant concentrations or when amorphous organic matter dominates sorption, measurements of soil foc and empirical correlations with contaminant properties appear to provide Kd estimates within about a factor of 3 of values measured in laboratory isotherms[7]. However, when TACMs are present and contaminant concentrations are low, the empirical correlations may substantially underestimate sorption. Additional research is needed to determine when simple empirical correlations may be used to estimate Kd and when more rigorous experimental investigations are required.
Summary
Nonionic and nonpolar organic contaminants including chlorinated solvents and petroleum hydrocarbons can become associated with solid phase organic matter in the soil, sediment, and rock. When amorphous organic matter is the primary sorbent, sorption is linear and distribution coefficients can be estimated from empirical correlations with contaminant properties (aqueous solubility or octanol-water partition coefficient) and site-specific measurements of solid phase organic carbon. However, when thermally altered carbonaceous materials (TACMs) are present, sorption may be non-linear and the empirical correlations may substantially underestimate sorption at low contaminant concentrations.
References
- ^ Piwoni, M.D. and Keeley, J.W., 1990. Basic concepts of contaminant sorption at hazardous waste sites. Ground-Water Issue; EPA-540/4-90/053. Report.pdf
- ^ 2.0 2.1 Chiou, C.T., Porter, P.E. and Schmedding, D.W., 1983. Partition equilibriums of nonionic organic compounds between soil organic matter and water. Environmental Science & Technology, 17(4), pp.227-231. doi: 10.1021/es00110a009
- ^ Ball, W.P. and Roberts, P.V., 1991. Long-term sorption of halogenated organic chemicals by aquifer material. 2. Intraparticle diffusion. Environmental Science & Technology, 25(7), pp.1237-1249. doi:10.1021/es00019a003
- ^ 4.0 4.1 Schwarzenbach, R.P. and Westall, J., 1981. Transport of nonpolar organic compounds from surface water to groundwater. Laboratory sorption studies. Environmental Science & Technology, 15(11), pp.1360-1367. doi: 10.1021/es00093a009
- ^ Karickhoff, S.W., 1981. Semi-empirical estimation of sorption of hydrophobic pollutants on natural sediments and soils. Chemosphere, 10(8), pp.833-846. doi:10.1016/0045-6535(81)90083-7
- ^ Chiou, C.T., Peters, L.J. and Freed, V.H., 1979. A physical concept of soil-water equilibria for nonionic organic compounds. Science, 206(4420), pp.831-832. doi: 10.1126/science.206.4420.831
- ^ 7.0 7.1 7.2 7.3 7.4 Allen-King, R.M., Grathwohl, P. and Ball, W.P., 2002. New modeling paradigms for the sorption of hydrophobic organic chemicals to heterogeneous carbonaceous matter in soils, sediments, and rocks. Advances in Water Resources, 25(8), pp.985-1016. doi: 10.1016/S0309-1708(02)00045-3
- ^ 8.0 8.1 Grathwohl, P., 1990. Influence of organic matter from soils and sediments from various origins on the sorption of some chlorinated aliphatic hydrocarbons: implications on Koc correlations. Environmental Science & Technology, 24(11), pp.1687-1693. doi: 10.1021/es00081a010
- ^ 9.0 9.1 Cornelissen, G., Kukulska, Z., Kalaitzidis, S., Christanis, K. and Gustafsson, Ö., 2004. Relations between environmental black carbon sorption and geochemical sorbent characteristics. Environmental Science & Technology, 38(13), pp.3632-3640. doi: 10.1021/es0498742
- ^ 10.0 10.1 Huang, W., Peng, P.A., Yu, Z. and Fu, J., 2003. Effects of organic matter heterogeneity on sorption and desorption of organic contaminants by soils and sediments. Applied Geochemistry, 18(7), pp.955-972. doi: 10.1016/S0883-2927(02)00205-6
- ^ Kleineidam, S., Rügner, H., Ligouis, B. and Grathwohl, P., 1999. Organic matter facies and equilibrium sorption of phenanthrene. Environmental Science & Technology, 33(10), pp.1637-1644. doi: 10.1021/es9806635
- ^ Binger, C.A., Martin, J.P., Allen-King, R.M. and Fowler, M., 1999. Variability of chlorinated-solvent sorption associated with oxidative weathering of kerogen. Journal of Contaminant Hydrology, 40(2), pp.137-158. doi: 10.1016/S0169-7722(99)00047-9