Difference between revisions of "User:Jhurley/sandbox"
| Line 8: | Line 8: | ||
* [[PFAS Transport and Fate]] | * [[PFAS Transport and Fate]] | ||
| − | '''Contributor(s):''' | + | '''Contributor(s):''' [[Dr. Sheau-Yun (Dora) Chiang]] and [[Dr. Alexandra Salter-Blanc]] |
'''Key Resource(s):''' | '''Key Resource(s):''' | ||
| − | *[https:// | + | *[https://pfas-1.itrcweb.org/wp-content/uploads/2020/04/ITRC_PFAS_TechReg_April2020.pdf Per- and Polyfluoroalkyl Substances (PFAS), PFAS-1. ITRC 2020.]<ref name="ITRC2020"/> |
| − | ==Introduction== | + | ==Introduction== |
| + | [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS)]] are a complex family of more than 3,000 manmade fluorinated organic chemicals<ref name="Wang2017">Wang, Z., DeWitt, J.C., Higgins, C.P., and Cousins, I.T., 2017. A Never-Ending Story of Per- and Poly-Fluoroalkyl Substances (PFASs)? Environmental Science and Technology, 51(5), pp. 2508-2518. [https://doi.org/10.1021/acs.est.6b04806 DOI: 10.1021/acs.est.6b04806] [[Media: Wang2017.pdf | Open access article.]]</ref> although not all of these are currently in use or production. PFAS are produced using several different processes. Fluorosurfactants, which include perfluoroalkyl acids (PFAAs) (see [[Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) | PFAS]] article for nomenclature) and side-chain fluorinated polymers, have been manufactured using two major processes: [[Wikipedia: Electrochemical fluorination | electrochemical fluorination (ECF)]] and [[Wikipedia: Telomerization | telomerization]] | ||
| + | |||
| + | |||
| + | |||
[[File:NewellMatrixDiffFig1.PNG | thumb |500px| Figure 1. Diffusion of a dissolved solute (chlorinated solvent) into lower ''K'' zones during loading period, followed by diffusion back out into higher ''K'' zones once the source is removed <ref name="Sale2007">Sale, T.C., Illangasekare, T.H., Zimbron, J., Rodriguez, D., Wilking, B., and Marinelli, F., 2007. AFCEE Source Zone Initiative. Air Force Center for Environmental Excellence, Brooks City-Base, San Antonio, TX. [https://www.enviro.wiki/images/0/08/AFCEE-2007-Sale.pdf Report.pdf]</ref>]] | [[File:NewellMatrixDiffFig1.PNG | thumb |500px| Figure 1. Diffusion of a dissolved solute (chlorinated solvent) into lower ''K'' zones during loading period, followed by diffusion back out into higher ''K'' zones once the source is removed <ref name="Sale2007">Sale, T.C., Illangasekare, T.H., Zimbron, J., Rodriguez, D., Wilking, B., and Marinelli, F., 2007. AFCEE Source Zone Initiative. Air Force Center for Environmental Excellence, Brooks City-Base, San Antonio, TX. [https://www.enviro.wiki/images/0/08/AFCEE-2007-Sale.pdf Report.pdf]</ref>]] | ||
Matrix Diffusion can have major impacts on solute migration in groundwater and on cleanup time following source removal. As a groundwater plume advances downgradient, dissolved contaminants are transported by [[Wikipedia: Molecular diffusion | molecular diffusion]] from zones with larger hydraulic conductivity (''K'') into lower ''K'' zones, slowing the rate of contaminant migration in the high ''K'' zone. However, once the contaminant source is eliminated, contaminants diffuse out of low ''K'' zones, slowing the cleanup rate in the high ''K'' zone (Figure 1). This process, termed ‘back diffusion’, can greatly extend cleanup times. | Matrix Diffusion can have major impacts on solute migration in groundwater and on cleanup time following source removal. As a groundwater plume advances downgradient, dissolved contaminants are transported by [[Wikipedia: Molecular diffusion | molecular diffusion]] from zones with larger hydraulic conductivity (''K'') into lower ''K'' zones, slowing the rate of contaminant migration in the high ''K'' zone. However, once the contaminant source is eliminated, contaminants diffuse out of low ''K'' zones, slowing the cleanup rate in the high ''K'' zone (Figure 1). This process, termed ‘back diffusion’, can greatly extend cleanup times. | ||
Revision as of 13:21, 28 January 2021
PFAS Sources
Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) have been used in coatings for textiles, paper products, and cookware; in some firefighting foams; and have a range of applications in the aerospace, photographic imaging, semiconductor, automotive, construction, electronics, and aviation industries[1][2][3]. Although PFAS and PFAS-containing products have been manufactured since the 1950s, PFAS were not widely documented in environmental samples until the early 2000s. Understanding PFAS manufacturing history, past and current uses, and waste management over the last six to seven decades is necessary for the identification of potential environmental sources of PFAS, possible release mechanisms, and associated pathway-receptor relationships.
Related Article(s):
Contributor(s): Dr. Sheau-Yun (Dora) Chiang and Dr. Alexandra Salter-Blanc
Key Resource(s):
Introduction
Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) are a complex family of more than 3,000 manmade fluorinated organic chemicals[4] although not all of these are currently in use or production. PFAS are produced using several different processes. Fluorosurfactants, which include perfluoroalkyl acids (PFAAs) (see PFAS article for nomenclature) and side-chain fluorinated polymers, have been manufactured using two major processes: electrochemical fluorination (ECF) and telomerization
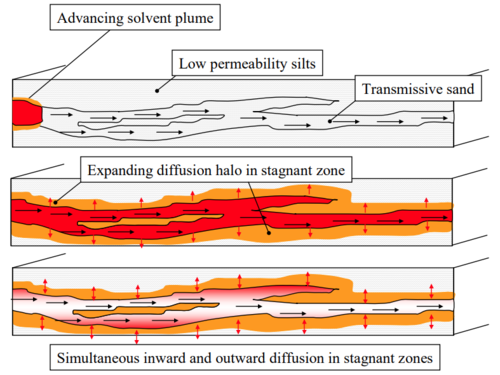
Matrix Diffusion can have major impacts on solute migration in groundwater and on cleanup time following source removal. As a groundwater plume advances downgradient, dissolved contaminants are transported by molecular diffusion from zones with larger hydraulic conductivity (K) into lower K zones, slowing the rate of contaminant migration in the high K zone. However, once the contaminant source is eliminated, contaminants diffuse out of low K zones, slowing the cleanup rate in the high K zone (Figure 1). This process, termed ‘back diffusion’, can greatly extend cleanup times.
The impacts of back diffusion on aquifer cleanup have been examined in controlled laboratory experiments by several investigators[6][7][8][9]. The video in Figure 2 shows the results of a 122-day tracer test in a laboratory flow cell (sand tank)[6]. The flow cell contained several clay zones (K = 10-8 cm/s) surrounded by sand (K = 0.02 cm/s). During the loading period, water containing a green fluorescent tracer migrated from left to right with the water flowing through the flow cell, while also diffusing into the clay. After 22 days, the fluorescent tracer is eliminated from the feed, and most of the green tracer is quickly flushed from the tank’s sandy zones. However, small amounts of tracer continue to diffuse out of the clay layers for over 100 days. This illustrates how back diffusion of contaminants out of low K zones can maintain low contaminant concentrations long after the contaminant source as been eliminated.
In some cases, matrix diffusion can maintain contaminant concentrations in more permeable zones above target cleanup goals for decades or potentially even centuries after the primary sources have been addressed. At a site impacted by Dense Non-Aqueous Phase Liquids (DNAPL), trichloroethene (TCE) concentrations in downgradient wells declined by roughly an order-of-magnitude (OoM) when the upgradient source area was isolated with sheet piling. However, after this initial decline, TCE concentrations appeared to plateau or decline more slowly, consistent with back diffusion from an underlying aquitard. Numerical simulations indicated that back diffusion would cause TCE concentrations in downgradient wells at the site to remain above target cleanup levels for centuries[10].
One other implication of matrix diffusion is that plume migration is attenuated by the loss of contaminants into low permeability zones, leading to slower plume migration compared to a case where no matrix diffusion occurs. This phenomena was observed as far back as 1985 when Sudicky et al. observed that “A second consequence of the solute-storage effect offered by transverse diffusion into low-permeability layers is a rate of migration of the frontal portion of a contaminant in the permeable layers that is less than the groundwater velocity.”[11] In cases where there is an attenuating source, matrix diffusion can also reduce the peak concentrations observed in downgradient monitoring wells. The attenuation caused by matrix diffusion may be particularly important for implementing Monitored Natural Attenuation (MNA) for contaminants that do not completely degrade, such as heavy metals and Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS).
SERPD/ESTCP Research
The SERDP/ESTCP programs have funded several projects focusing on how matrix diffusion can impede progress towards reaching site closure, including: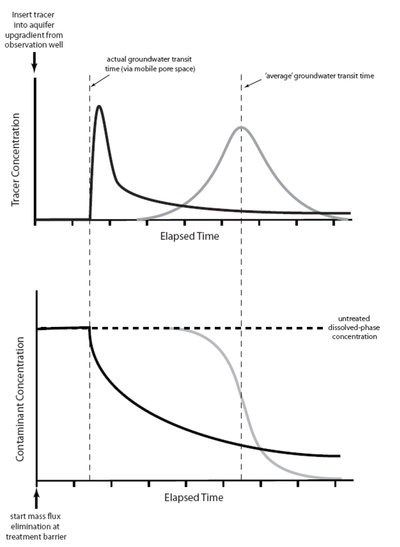
Transport Modeling
Several different modeling approaches have been developed to simulate the diffusive transport of dissolved solutes into and out of lower K zones[17][18]. The Matrix Diffusion Toolkit[13] is a Microsoft Excel based tool for simulating forward and back diffusion using two different analytical models[19][20]. Numerical models including MODFLOW/MT3DMS[21] have been shown to be effective in simulating back diffusion processes and can accurately predict concentration changes over 3 orders-of-magnitude in heterogeneous sand tank experiments[22]. However, numerical models require a fine vertical discretization with short time steps to accurately simulate back diffusion, greatly increasing computation times[23]. These issues can be addressed by incorporating a local 1-D model domain within a general 3D numerical model[24].
The REMChlor - MD toolkit is capable of simulating matrix diffusion in groundwater contaminant plumes by using a semi-analytical method for estimating mass transfer between high and low permeability zones that provides computationally accurate predictions, with much shorter run times than traditional fine grid numerical models[15].
Impacts on Breakthrough Curves
The impacts of matrix diffusion on the initial breakthrough of the solute plume and on later cleanup are illustrated in Figure 3[16]. Using a traditional advection-dispersion model, the breakthrough curve for a pulse tracer injection appears as a bell-shaped (Gaussian) curve (gray line on the right side of the upper graph) where the peak arrival time corresponds to the average groundwater velocity. Using an advection-diffusion approach, the breakthrough curve for a pulse injection is asymmetric (solid black line) with the peak tracer concentration arriving earlier than would be expected based on the average groundwater velocity, but with a long extended tail to the flushout curve.
The lower graph shows the predicted cleanup concentration profiles following complete elimination of a source area. The advection-dispersion model (gray line) predicts a clean-water front arriving at a time corresponding to the average groundwater velocity. The advection-diffusion model (black line) predicts that concentrations will start to decline more rapidly than expected (based on the average groundwater velocity) as clean water rapidly migrates through the highest-permeability strata. However, low but significant contaminant concentrations linger much longer (tailing) due to diffusive contaminant mass exchange between zones of high and low permeability. A similar response to source remediation is seen in models such as the sand tank experiment shown in Figure 2, and also in field observations of plume contaminant concentrations in heterogeneous aquifers.
References
- ^ 1.0 1.1 Interstate Technology and Regulatory Council (ITRC), 2020. Technical/Regulatory Guidance: Per- and Polyfluoroalkyl Substances (PFAS), PFAS-1. ITRC, PFAS Team, Washington DC. Website: https://pfas-1.itrcweb.org/ Free Download from ITRC. Report.pdf
- ^ Swedish Chemicals Agency (KEMI), 2015. Occurrence and use of highly fluorinated substances and alternatives, Report 7/15. ISSN 0284-1185. Article number 361 164. Report.pdf
- ^ US Environmental Protection Agency (USEPA), 2021. Basic Information on PFAS. Website
- ^ Wang, Z., DeWitt, J.C., Higgins, C.P., and Cousins, I.T., 2017. A Never-Ending Story of Per- and Poly-Fluoroalkyl Substances (PFASs)? Environmental Science and Technology, 51(5), pp. 2508-2518. DOI: 10.1021/acs.est.6b04806 Open access article.
- ^ Sale, T.C., Illangasekare, T.H., Zimbron, J., Rodriguez, D., Wilking, B., and Marinelli, F., 2007. AFCEE Source Zone Initiative. Air Force Center for Environmental Excellence, Brooks City-Base, San Antonio, TX. Report.pdf
- ^ 6.0 6.1 Doner, L.A., 2008. Tools to resolve water quality benefits of upgradient contaminant flux reduction. Master’s Thesis, Department of Civil and Environmental Engineering, Colorado State University.
- ^ Yang, M., Annable, M.D. and Jawitz, J.W., 2015. Back Diffusion from Thin Low Permeability Zones. Environmental Science and Technology, 49(1), pp. 415-422. DOI: 10.1021/es5045634 Free download available from: ResearchGate
- ^ Yang, M., Annable, M.D. and Jawitz, J.W., 2016. Solute source depletion control of forward and back diffusion through low-permeability zones. Journal of Contaminant Hydrology, 193, pp. 54-62. DOI: 10.1016/j.jconhyd.2016.09.004 Free download available from: ResearchGate
- ^ Tatti, F., Papini, M.P., Sappa, G., Raboni, M., Arjmand, F., and Viotti, P., 2018. Contaminant back-diffusion from low-permeability layers as affected by groundwater velocity: A laboratory investigation by box model and image analysis. Science of The Total Environment, 622, pp. 164-171. DOI: 10.1016/j.scitotenv.2017.11.347
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedChapman2005 - ^ Sudicky, E.A., Gillham, R.W., and Frind, E.O., 1985. Experimental Investigation of Solute Transport in Stratified Porous Media: 1. The Nonreactive Case. Water Resources Research, 21(7), pp. 1035-1041. DOI: 10.1029/WR021i007p01035
- ^ Cite error: Invalid
<ref>tag; no text was provided for refs namedSale2013 - ^ 13.0 13.1 Farhat, S.K., Newell, C.J., Seyedabbasi, M.A., McDade, J.M., Mahler, N.T., Sale, T.C., Dandy, D.S. and Wahlberg, J.J., 2012. Matrix Diffusion Toolkit. Environmental Security Technology Certification Program (ESTCP) Project ER-201126. User’s Manual.pdf Website: ER-201126
- ^ Sale, T. and Newell, C., 2011. A Guide for Selecting Remedies for Subsurface Releases of Chlorinated Solvents. Environmental Security Technology Certification Program (ESTCP) Project ER-200530. Report.pdf Website: ER-200530
- ^ 15.0 15.1 Farhat, S. K., Newell, C. J., Falta, R. W., and Lynch, K., 2018. A Practical Approach for Modeling Matrix Diffusion Effects in REMChlor. Environmental Security Technology Certification Program (ESTCP) Project ER-201426. User’s Manual.pdf Website: ER-201426
- ^ 16.0 16.1 Interstate Technology and Regulatory Council (ITRC), 2011. Integrated DNAPL Site Strategy (IDSS-1), Integrated DNAPL Site Strategy Team, ITRC, Washington, DC. Report.pdf Free download from: ITRC
- ^ Falta, R.W., and Wang, W., 2017. A semi-analytical method for simulating matrix diffusion in numerical transport models. Journal of Contaminant Hydrology, 197, pp. 39-49. DOI: 10.1016/j.jconhyd.2016.12.007
- ^ Muskus, N. and Falta, R.W., 2018. Semi-analytical method for matrix diffusion in heterogeneous and fractured systems with parent-daughter reactions. Journal of Contaminant Hydrology, 218, pp. 94-109. DOI: 10.1016/j.jconhyd.2018.10.002
- ^ Parker, B.L., Gillham, R.W., and Cherry, J.A., 1994. Diffusive Disappearance of Immiscible Phase Organic Liquids in Fractured Geologic Media. Groundwater, 32(5), pp. 805-820. DOI: 10.1111/j.1745-6584.1994.tb00922.x
- ^ Sale, T.C., Zimbron, J.A., and Dandy, D.S., 2008. Effects of reduced contaminant loading on downgradient water quality in an idealized two-layer granular porous media. Journal of Contaminant Hydrology, 102(1), pp. 72-85. DOI: 10.1016/j.jconhyd.2008.08.002
- ^ Zheng, C. and Wang, P.P., 1999. MT3DMS: A Modular Three-Dimensional Multispecies Transport Model for Simulation of Advection, Dispersion, and Chemical Reactions of Contaminants in Groundwater Systems; Documentation and User’s Guide. Contract Report SERDP-99-1 U.S. Army Engineer Research and Development Center, Vicksburg, MS. User’s Guide.pdf MT3DMS website
- ^ Chapman, S.W., Parker, B.L., Sale, T.C., Doner, L.A., 2012. Testing high resolution numerical models for analysis of contaminant storage and release from low permeability zones. Journal of Contaminant Hydrology, 136, pp. 106-116. DOI: 10.1016/j.jconhyd.2012.04.006
- ^ Farhat, S.K., Adamson, D.T., Gavaskar, A.R., Lee, S.A., Falta, R.W. and Newell, C.J., 2020. Vertical Discretization Impact in Numerical Modeling of Matrix Diffusion in Contaminated Groundwater. Groundwater Monitoring and Remediation, 40(2), pp. 52-64. DOI: 10.1111/gwmr.12373
- ^ Carey, G.R., Chapman, S.W., Parker, B.L. and McGregor, R., 2015. Application of an Adapted Version of MT3DMS for Modeling Back‐Diffusion Remediation Timeframes. Remediation, 25(4), pp. 55-79. DOI: 10.1002/rem.21440
See Also
- Matrix Diffusion Movie
- Impact of Clay-DNAPL Interactions on Transport and Storage of Chlorinated Solvents in Low Permeability Zones
- Prediction of Groundwater Quality Improvement Down-Gradient of In Situ Permeable Treatment Barriers and Fully Remediated Source Zones
- Determining Source Attenuation History to Support Closure by Natural Attenuation
- Coursera Matrix Diffusion Online Lecture